Top Links
Journal of Plant Sciences and Crop Protection
ISSN: 2639-3336
Bioactivity of Plant Extracts Against (Fusarium Oxysporum f. sp. Lycopersici Sacc.)Causing Wilt Disease of Tomato (Solanum Lycopersicum L) in the Southern Bioactivity of Plant Extracts Against Fusarium Oxysporum f. sp. Lycopersici Sacc.) Causing Wilt Disease of Tomato (Solanum Lycopersicum L) in the Southern Guinea Savannah, Nigeria Guinea Savannah, Nigeria
Copyright: © 2024 Gwa VI. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Wilt disease of tomato is caused by Fusarium oxysporum f. sp. lycopersici and it is an important disease which causes significant yield reduction in the crop throughout the world.
Keywords: Fusarium oxysporum f. sp. lycopersici; Fusarium wilt; Inhibition; Pathogenicity, Plant extract, Mancozeb; Tomato
Several diseases threaten tomato (Lycopersicon esculentum L.) both during production and after harvest, reducing the yield and causing financial losses. Anthracnose, blight, and wilt are the three main tomato diseases [1-3]. A deadly soil-borne wilt disease caused by Fusarium oxysporum f. sp. lycopersici is the most significant, widespread and destructive disease that infects and reduces the economic value of tomato and other ornamental crops [4-6]. The fungus enters the plant through its roots and spreads to the xylem vessels, which are the vascular tissues. The fungus obstructs the movement of water and nutrients as it invades the vascular tissues. Due to the lack of water, the stomata on the leaves close, causing the plant to exhibit symptoms like gradual wilting, progressive yellowing of the leaves, collapse at the petiole, and eventual plant death [5]. Infected plants typically display symptoms like chlorosis, wilting of leaves, and browning of the vascular system. The plant presents notable typical internal symptoms, such as the characteristic light yellow to dark brown discoloration of vascular tissues. This starts off in the older or lower leaf and then spreads upward to the newer or upper leaf.
Tomato fruits are particularly perishable and vulnerable to pathogenic infection both in the field and during postharvest harsh handling due to their high water content. Fruit rots are typically caused by fungi and bacteria [7, 8, 9]. Some of the fungi and bacteria linked to diseases in tomato are Alternaria solani, Aspergillus niger, Botrytis ceneria, Curvularia spp., Colletotrichum spp., F. moniliformis, F. oxysporum, Geotrichum spp., Penicillium spp., Phytophthora spp., Mucor spp., Rhizopus stolonifer and Erwinia spp. [8-14].
Postharvest losses from tomato have been estimated to reach up to 25.80%, depending on the interactions of the host, pathogen and environmental factors [15]. It has been suggested that various pesticides including synthetic chemicals, biological control techniques, and botanicals be used to manage these pathogens [16]. When it comes to controlling crop diseases, chemical pesticides are typically more dependable and efficient [17]. However, the synthetic chemicals are non-biodegradable, non-target specific, have a lengthy residual effect, and are hazardous to living things, including humans, animals, plants, soil, and water bodies [18]. Many biological control agents including Trichoderma species, Pseudomonas syringae and Pseudomonas chlororaphis have proven to be helpful in managing Fusarium wilt and other pathogens [19-22].
Studies conducted in many parts of the world on plant fungicides have led researchers to conclude that plants are effective in the management of phytopathogens even without the use of synthetic pesticides [23-27]. This is due to the fact that plant materials are highly biodegradable, inexpensive, ecologically friendly, and readily available in managing plant pathogenic fungi [14, 28, 29]. According to [30], the phytochemicals found in these plants have two main effects: they can directly affect the pathogen or cause the host to acquire systemic resistance which slows the progression of the disease.
Alkaloids, flavonoids, phenols, terpenoids, tannins, and other compounds are examples of secondary metabolites in plants that function as chemical defense against plant pathogenic fungi [31, 32]. The objectives of the study were to look at methods other than synthetic chemicals for managing F. oxysporum f. sp. lycopersici which causes tomato wilt disease in the region. The study therefore, sought to investigate the fungicidal and fungistatic potentials of three commonly used botanicals, Azadirachta indica, Piper guineense, and Zingiber officinale, either applied alone or in combination with each other against F. osysporum; compare the effect of each botanical with the synthetic chemical, mancozeb on the radial growth of F. osysporum; and to determine their effect on postharvest losses associated with the pathogen.
This work was carried out in the Plant Pathology Laboratory, Federal University of Agriculture, Makurdi, Nigeria in 2016.
Infected roots, stems, leaves, and fruits of tomato plants exhibiting the characteristic symptoms of Fusarium wilt were collected from different tomato farms in Tarka Local Government Area, Benue State, Nigeria. The samples were aseptically packed in sterile polythene bags and taken to the Laboratory for additional research.
At the interphase between the healthy and infected portions of the samples, diseased tomato plants exhibiting pronounced typical symptoms of wilt were chopped into small pieces, about 2×2 mm in diameter, and washed three times under running tap water. The cut samples were submerged for about 30 seconds in 5% sodium hypochlorite solution [11]. In order to eliminate any extra Clorox, which would have prevented the pathogen's growth, the sterilized plant pieces were immersed in three separate changes of sterile water. The plant pieces were placed on sterile paper and allowed to blot dry for about ten minutes prior to inoculation.
Potato dextrose agar (PDA), prepared as recommended by the manufacturer, was poured in sterilized Petri dishes. The medium was amended with 0.16 g of powdered streptomycin sulphate to inhibit the growth of bacteria before being poured into Petri plates [17]. For the inoculation, 20 mL of PDA were put into 90 cm plates and left to harden. Four pieces each of the infected fruit, root, leaf and stem tissues were aseptically and separately plated at equal spacing from each other in the plates. The plates were incubated for seven days at 30 ± 5 0 C. The frequency of occurrence of F. oxysporum f. sp. lycopersici on the fruit, stem, leaf, and root samples was determined by routinely examining the growing colonies of each section of the plant tissues.
Fusarium oxysporum f. sp. lycopersici was identified based on the cultural traits of the fungus cultivated on PDA. A compound microscope was used to determine the morphological (size and form of macro and micro conidia) and cultural (mycelial colour) features of interest [33]. The fungal identity was cross-referenced with the industry standard guide for F. oxysporum identification [34].
The percentage frequency of occurrence of F. oxysporum isolates was determined using the method of [27]. This was based on the number of times the F. oxysporum pathogen was isolated from various parts of infected tomato plants relative to the total number of F. oxysporum isolated on all parts of the tomato at a given time.
Where,
p = number of times an isolate occurred on a particular part of tomato at a given time
q = the overall number of times the isolate occurred on all parts of the tomato at a given time.
A pathogenicity test was conducted on tomato plants using isolates of F. oxysporum. Two seedlings of 21-day-old tomato transplants were placed in 30 cm-diameter polythene bags containing 10 kg of sterilized soil. A suspension of 106 spores/ml of F. oxysporum isolate was made, and 20 mL were applied to each seedling. Each plant stand in the control received 20 mL of distilled water; there were three replications. The plants were watered regularly for four weeks after inoculation to allow for the development of wilt symptoms. The infected tomato fruits, roots, stem, and leaves, as well as the uninoculated plants exhibiting different degrees of symptoms were chopped and cultured for seven days on PDA to observe the fungal growth. Following re-isolation, growth characteristics were noted and a comparison was done between the original field-derived culture and the artificially inoculated culture.
The method of [27] was adopted for this research work for the preparation of plant extracts. The concentrations of 40, 80 and 120 g/L of M. oleifera and Z. officinale previously demonstrated [27] proved effective against A. niger and A. flavus of groundnut in vitro. This necessitated the use of these concentrations against F. oxysporum of tomato in vitro. Powdered A. indica leaves, P. guineense seeds, and Z. officinale rhizomes, each at 40 g, 80 g, and 120 g were measured with an electric weighing machine and separately added to 1000 mL conical flasks filled with sterile water. The volume of the water was measured with a 1000 mL measuring cylinder and autoclaved at 100 o C. The mixtures were vigorously stirred and allowed to settle for 24 hours before being filtered through three layers of muslin cloth. Concentrations of 40, 80, and 120 g/L of extracts of A. indica leaves, P. guineense seeds, and Z. officinale rhizomes were produced. Precisely 6 mL of each of the resulting plant extract combinations depending on the treatments at different concentrations were added to 14 mL of PDA to make up the required 20 mL of PDA in the plates prior to inoculating F. oxysporum. In the control treatment (0 g/L), 6 mL of distilled water were added in the place of the plant extract. All plates were incubated for seven days at room temperature.
Using a transparent ruler, the growth of F. oxysporum f. sp. lycopersici was measured every 24 hours for 120 hours, and mean values were computed. Fungal toxicity was measured using the percentage growth inhibition (PGI) technique, which was first presented by [35] and more recently by [16].
Where;
PGI = Percentage Growth Inhibition
R = Fungal growth in the control plate.
R1 = Fungal growth in the treated plate
The research covers botanicals of economic importance in the study area and as such can be easily accessed by the farmers. Tomato is commonly grown in the study area and F. oxysporum is a pathogen which affects its production. Controlling the fungus with locally available plants is of economic benefit to the farmers. One of the challenges is that F. oxyporum may coexist with other pathogens and the infection may not necessarily be associated with the pathogen alone.
Fusarium oxysporum f. sp. lycopersici was grown on PDA and the treatments (different concentrations of extracts of the plants, A. indica, P. guineense, and Z. officinale, and Mancozeb, a synthetic fungicide), were employed to suppress it. To ascertain their bioactive effects on the tomato wilt fungus in vitro, the treatments were applied singly or in combination. In general, 14 mL of PDA were added to a total of 6 mL of each treatment applied singly or as a combination of two or three, thoroughly mixed, allowed to set, and the fungus was inoculated. The treatment combinations were as follows.
The data collected from the various treatments were analyzed using GenStat Discovery Edition 12 for ANOVA, Graph Pad Prism 6 for trend graphs, and Fisher's least significant difference (FLSD) (P≤0.05) to separate the significant means for each measured parameter [36].
Fusarium oxysporum was identified based on its fast growth rate on PDA with a range of colour changes in the aerial mycelial growth beginning from white to peach, salmon, wine grey to purple or violet producing a thick matted mycelial growth as seen in Figure 1a. There were abundant macroconidia, hyaline, single-celled, variable and oval to kidney shaped. The macroconidia are thin walled, curved and pointed at both ends, bearing 3-7 septa with hooked apex and foot-shaped basal end as presented in Figure 1b.
Fusarium oxysporum f. sp. lycopersici isolates were obtained from the roots, fruits, stems, and leaves of tomato plants during the period under investigation. Table 1 shows the frequency and percentage frequency of isolates of the fungus from the various parts of tomato. The results revealed that the highest percentage frequency of occurrence of the isolates was on the roots (35.56 %) followed by the stems (26.67 %) while the least was on the leaves (17.77 %).
Figure 2a shows the variation of disease symptoms on aerial parts and within the stem tissues of tomato plants inoculated with F. oxysporum f. sp. lycopersici. At the early stage of the disease, symptoms appeared as yellowing of the lower leaves and in the later stages, drooping of the leaves was observed. During severe infection, the pith of the stem turned brown in colour. Also, the lower leaves of severely infected plants dried up; ultimately the aerial parts of the plant showed loss of turgidity and drooped down. The uninoculated plants were not infected throughout the growth period and looked green as presented in Figure 2b.
Table 2 shows the degree of pathogenicity of F oxysporum inoculated into different parts of tomato plants with various symptoms of infection such as yellowing of leaves, wilting, and death of the plant. The results revealed that all the parts of tomato were infected and had varying degrees of symptoms. The uninoculated plants did not have wilt symptoms and were therefore classified as healthy.
Figure 3 shows the effect of various concentrations of A. indica extract on the growth of F. oxysporum isolated on tomato 120 hours after incubation (HAI). The results revealed that growth inhibition was more effective at a concentration of 120 g/L of the extract followed by 80 g/L while the least was obtained at 40 g/L of A. indica throughout the period of incubation.
The results show that when 3 mL of each of the concentrations of A. indica extract and mancozeb were amended separately in PDA, the growth inhibition was highest at 120 g/L. The interactions between A. indica extract and mancozeb increased the growth inhibition of F. oxysporum f. sp. lycopersici; the least percentage (76.0 %) was recorded at a concentration of 40 g/L, 24 HAI while the highest (91 %) was obtained at 120 g/L, 120 HAI (Figure 4). The steady increase in growth was as a result of the interactions between A. indica with mancozeb.
Figure 5 shows the interactions between A. indica and P. guineense at the different concentrations of the plant extracts. The results revealed that 120 g/L of the two extracts inhibited the growth of F. oxysporum slightly more than 80 g/L and 40 g/L. The least growth inhibition of F. oxysporum (58 %) was observed at 40 g/L while the highest (80 %) was obtained at 120 g/L. The results also indicated that growth was comparable at 24 and 48 HAI but decreased from 48 to 72 HAI.
The results presented in Figure 6 show the interactions between different concentrations of A. indica, P. guineense and mancozeb on percentage growth inhibition of F. oxysporum f. sp. lycopersici at various times of incubation. The results revealed that the level of inhibition increased from 24 to 48 HAI and decreased steadily from 48 to 120 HAI. At 48 HAI, a combination of extracts of the two plants and mancozan each at a concentration of 120 g/L produced the highest growth inhibition (90 %); this was closely followed by 80 g/L (87 %) and then 40 g/L (84 %). At 24 HAI, the interactions between the plant extracts and mancozan had the least inhibitory effect at each of the concentrations.
Results presented in Figure 7 show the interactions of extracts of A. indica, P. guineense and Z. officinale at various concentrations. The results indicated that growth inhibition of F. oxysporum increased from 24 to 48 HAI and decreased sharply from 48 to 96 HAI before increasing gradually from 96 to 120 HAI. This trend was observed for the different concentrations of the three plant extracts; the concentration of 120 g/L had the highest inhibitory effect followed by 80 g/L and lastly 40 g/L. The percentage of growth inhibition of the fungus decreased with increase in the duration of incubation from 48 to 120 HAI.
Figure 8 reveals the effect of interactions of A. indica and Z. officinale extracts at different concentrations on the growth inhibition of F. oxysporum. The results showed that the two plant extracts are closely related; the concentrations of 120 g/L and 80 g/L of both plant extracts differed slightly and gave higher fungal inhibition than 40 g/L throughout. The least inhibition (60%) was observed at a concentration of 40 g/L, 96 HAI while the highest (80%) was recorded at 120 g/L, 24 HAI.
Figure 9 shows the effect of interactions of the different concentrations of A. indica, Z. officinale and mancozeb on the growth inhibition of F. oxysporum. The results revealed that a concentration of 120 g/L of the plant extracts and mancozeb inhibited the growth of F. oxysporum more effectively than 40 and 80 g/L at 24 HAI. The results further showed that from 48 to 120 HAI, there were only slight differences among the various concentrations of both plant extracts and mancozeb.
Figure 10 shows the effect of different concentrations of P. guineense on growth inhibition of F. oxysporum after 120 hours of incubation. The results revealed that there was a slight increase in growth inhibition from 24 to 48 HAI at all the concentrations of the plant extract but this also decreased gradually from 48 to 120 HAI. This indicated that growth inhibition decreased with an increase in the duration of incubation irrespective of the concentration used.
The results presented in Figure 11 show that the inhibition of fungal growth increased steadily from 24 to 120 HAI at all the concentrations of Piper guineense extract and mancozeb. The fungal growth inhibition was better at a concentration of 120 g/L than at 80 and 40 g/L throughout the period of incubation. At 40 and 80 g/L, the inhibitory effect of the plant extract and fungicide was comparable from 72 to 120 HAI.
The effect of the interactions between P. guineense and Z. officinale at different concentrations on the inhibition of F. oxysporum growth is presented in Figure 12. The results indicated that fungal growth inhibition increased from 24 to 48 HAI and decreased gradually from 48 to 120 HAI. From 24 to 120 HAI, the extract of both plants had similar inhibitory effect against the fungus at the different concentrations. The results show that the two plants are closely related to each other in their potencies.
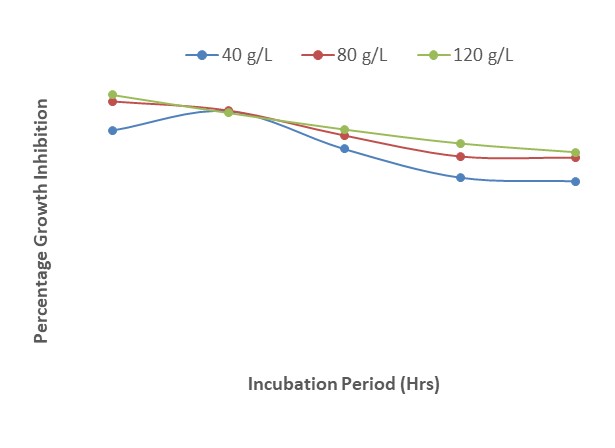
Figure 13 shows that fungal growth inhibition increased as the duration of incubation increased though there were only slight differences between the different concentrations of the plant extracts and mancozan. At a concentration of 120 g/L, the interactions of the plant extracts and fungicide appeared slightly more potent than at 40 and 80 g/L. The presence of mancozeb seemed to increase the percentage inhibition from 87 % at 24 HAI to 95 % at 120 HAI.
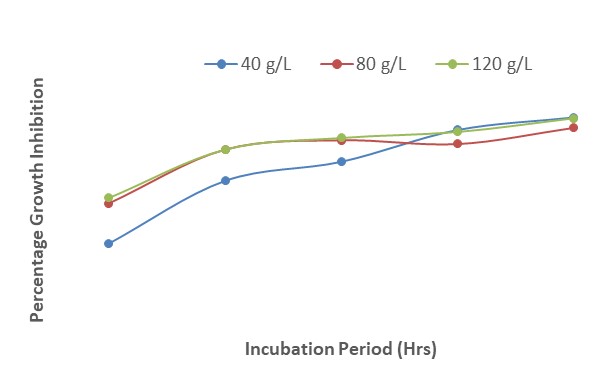
Figure 14 shows a gradual decrease in growth inhibition of F. oxysporum as a result of increase in the duration of incubation at the different concentrations of Z. officinale extract. The results also revealed that 40 g/L of the extract inhibited the growth of the pathogen by 70 % at 24 HAI but decreased to 65 % at 120 HAI. A similar trend was observed for 80 g/L and 120 g/L of the extract.
Figure 15 shows the interactions between Z. officinale and mancozeb on growth inhibition of F. oxysporum after 120 hours of incubation. The results revealed that at 40 g/L, there was a steady increase in fungal growth inhibition from 80 % at 24 HAI to 95% at 120 HAI. Similar results were recorded for 80 g/L and 120 g/L. The presence of mancozeb increased the potency of the mixture hence there was more inhibition in the growth of the pathogen.
Results presented in Table 3 show the mean variation of percentage growth inhibition of F. oxysporum at the different concentrations of plant extracts and mancozeb, a synthetic fungicide. The results revealed that at a concentration of 40 g/L, plant extracts applied alone were less potent in inhibiting the mycelia growth of F. oxysporum than those combined. The results further showed that the extract of A. indica alone inhibited growth of F. oxysporum by 36.99 % and this was lower than when combined either with P. guineense (64.55 %), Z. officinale (67.51 %), mancozeb (84.48 %) or all of them. Similarly, results of the application of P. guineense alone (52.53 %) were found to be significantly different (P< 0.05) from those obtained when the plant extract was applied with either A. indica or Z. officinale or mancozeb. This trend of results was observed using 80 g/L and 120 g/L of the different combinations of the plant extracts used. It was observed that mancozeb alone consistently gave better results at 40 g/L (94.34 %), 80 g/L (100 %) and 120 g/L (100 %) than any of the plant extracts when applied alone or combined with another plant extract or mancozeb. There was a significant difference (P< 0.05) among the treatments when compared at 80 g/L and 120 g/L. It was equally observed that the combination of mancozeb with any of the plant extracts was more potent than when the plant extracts were combined; this indicates that, mancozan increased the potency of the mixture. Generally, the mean percentage growth inhibition at a concentration of 80 g/L was least for A. indica alone (53.44 %) and highest when a combination of P. guineense × Z. officinale × mancozeb (92.07 %) was used. Similar results were obtained at 120 g/L; Azadirachta indica alone produced the least mean growth inhibition of 59.96 % compared to the highest mean value of 93.19 % when a combination of P. guineense × Z. officinale × mancozeb was applied.
This study indicated that Fusarium oxysporum f. sp. lycopersici is a pathogen of wilt disease of tomato which infects and colonizes the roots, stems, leaves and fruits of the plant. The fungus gained entrance into the plant through the roots and spread rapidly to the vascular tissues and xylem vessels. It has been reported that the fungus is the most devastating pathogen of tomato [6, 3, 9] and could cause a yield reduction of approximately 25.80% annually [15].
The pathogenicity test conducted on different parts of the plant showed that the pathogen incited the disease and it was more virulent in the roots than the other parts of the plant which also showed infection. The uninoculated parts of tomato however, did not produce symptoms of wilt and were therefore free of wilt disease. These results corroborate the findings of [5] that inoculated F. oxysporum strains on tomato and found a significant reduction in all growth parameters of the cultivars tested compared with the uninoculated plants
The researchers found the extracts effective in managing the pathogen and this may be attributed to the presence of phytochemical compounds such as alkaloids, coumarins, flavonoids, phenolic acids, quinines, tannins, and terpenoids in the plant materials as earlier reported by [37, 38, 39]. The mechanism of action against the pathogen could be interference with electron transport, cell permeability alteration, cellular wall interference, nutrient absorption, and deactivation of various cellular enzymes and denaturation of cellular proteins [40].
The combination of mancozeb with A. indica, P. guineense or Z. officinale at different concentrations proved more effective than when mancozeb was combined with any two plant fungicides after 120 hours of incubation. This is similar to the work of [41] which showed that various plant extracts and chemical fungicides have inhibitory effect against F. oxysporum f. sp. lycopersici; and that of [42] which showed that mancozeb + Thiophanate methyl (0.15 %) completely inhibited the radial growth of F. oxysporum after 168 hours of incubation.
Results showed that the plant extracts applied alone or in combination with other plant extracts or the synthetic fungicide mancozeb significantly inhibited mycelial growth of F. oxysporum in vitro. However, mancozeb alone (6 mL) consistently gave 100 % inhibition of growth of the pathogen at concentrations of 80 and 120 g/L. These results are similar to the earlier research carried out by [43] who indicated that mancozeb inhibited the growth of F. solani by 100 %. The researchers equally tested A. indica, P. guineense and Z. officinale extracts and found them effective against F. solani with growth inhibition of 52.71 % (30 g/L) to 63.94 % (90 g/L), 57.94 % (30 g/L) to 74.89 % (90 g/L) and 57.69 % (30 g/L) to 73.12 % (90 g/L) after 120 hours of incubation respectively..
Similarly, [44] reported that A. indica, P. guineense, Z. officinale and mancozeb inhibited the growth of A. niger The authors found that mancozeb consistently stopped the radial growth of the pathogen by 100 % throughout the period of incubation. In a related development, [11] evaluated the fungicidal effect of A. indica and Z. officinale extracts and found them effective in the management of F. oxysporum and Rhizoctonia solani isolated from fruits of tomato.
The report by [45] confirmed the fungicidal potency of Piper bettle against F. oxysporum f. sp. lycopersici. These results clearly showed that the plant extracts either alone or combined were effective in controlling F. oxysporum; however, mancozeb was the most effective in controlling the pathogen and equally increased the potency of the plant extracts when it was used in combination with them.
Fusarium oxysporum f. sp. lycopersici is the causal agent of Fusarium wilt disease of tomato. All the various organs (root, fruit, leaf, and stem) of tomato plants are susceptible to the pathogen at all stages of growth and the pathogen is more associated to the roots of the plant than the other parts. All the plant extracts and synthetic fungicide (A. indica, P. guineense, Z. officinale and mancozeb) at different concentrations were effective in controlling F. oxysporum f. sp. Lycopersici; a combination of any of these plant extracts with another one or mancozeb tended to be more effective than when applied alone. It was also found that, among the plant extracts; Z. officinale was most effective followed by P. guineense while the least was A. indica irrespective of the concentration used. The concentration of 120 g/L was more effective in inhibiting the growth of the pathogen than 80 g/L and 40 g/L in spite of the combination of treatments. It is therefore, recommended that the plant extracts be used either alone or combined with another plant extract or mancozeb to control F. oxysporum f. sp lycopersici, the causal pathogen of wilt disease of tomato.
The authors of this research article declare that there were no conflicts of interest
This research received no specific grant from any funding agency in the public, commercial or not-for- profit sectors.
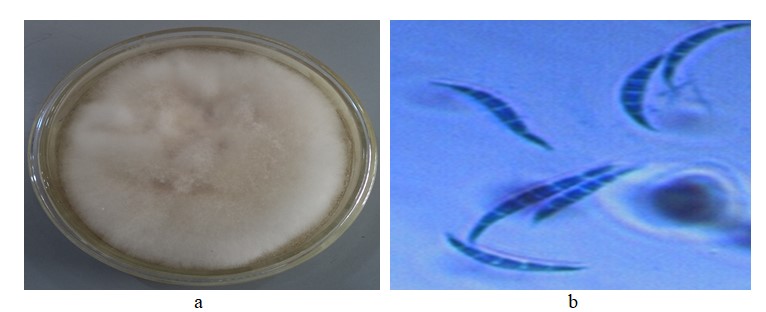 |
| Figure 1: Culture of Fusarium oxysporum f. sp. lycopersici grown on PDA (a) and Macroconidia of F. oxysporum f. sp. lycopersici (b) |
 |
| Figure 2: A cross section of tomato plants showing wilt infection caused by inoculation of F. oxysporum strains (a); uninoculated healthy tomato plant (b) |
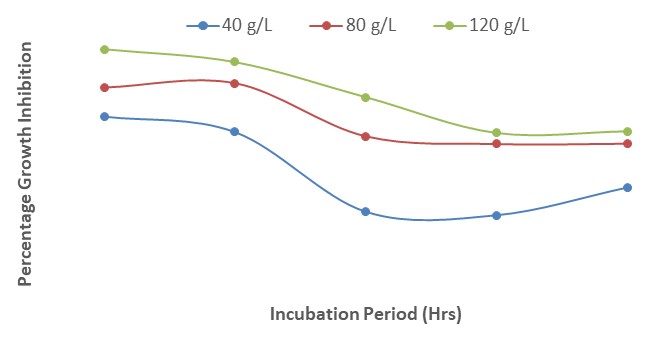 |
| Figure 3: Effect of different concentrations of Azadirachta indica on growth inhibition of Fusarium oxysporum f. sp. lycopersici after 120 hours of incubation |
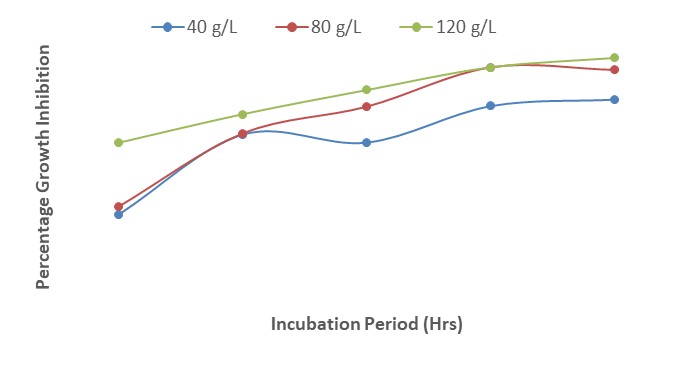 |
| Figure 4: Effect of interactions of different concentrations of Azadirachta indica and mancozeb on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
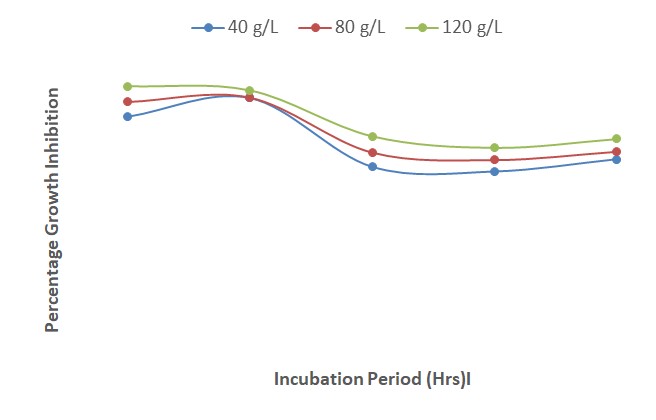 |
| Figure 5: Effect of interactions between different concentrations of Azadirachta indica and Piper guineense on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
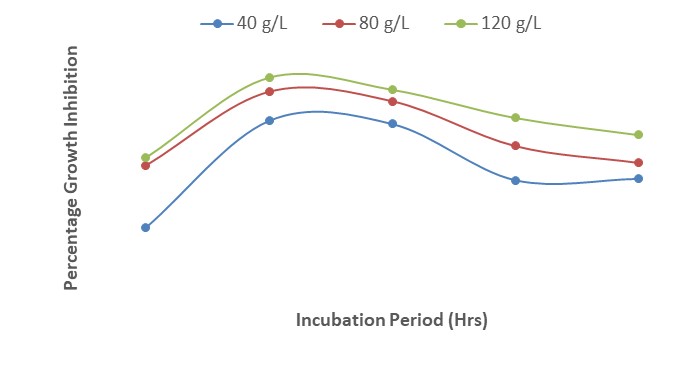 |
| Figure 6: Effect of interactions of different concentrations of Azadirachta Indica, Piper guineense and mancozeb on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
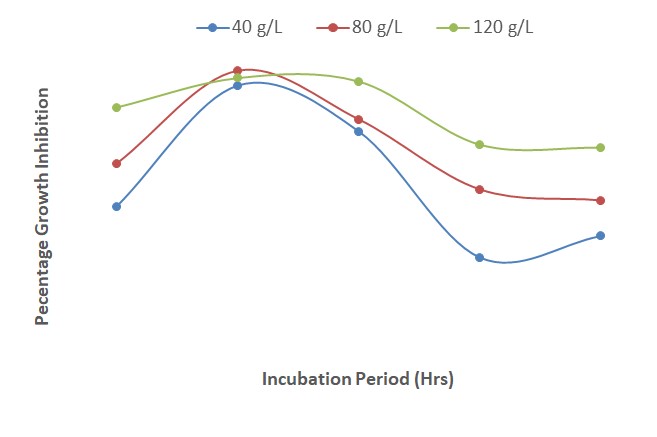 |
| Figure 7: Effect of interactions of different concentrations of Azadirachta indica x Piper guineense x Zingiber officinale on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
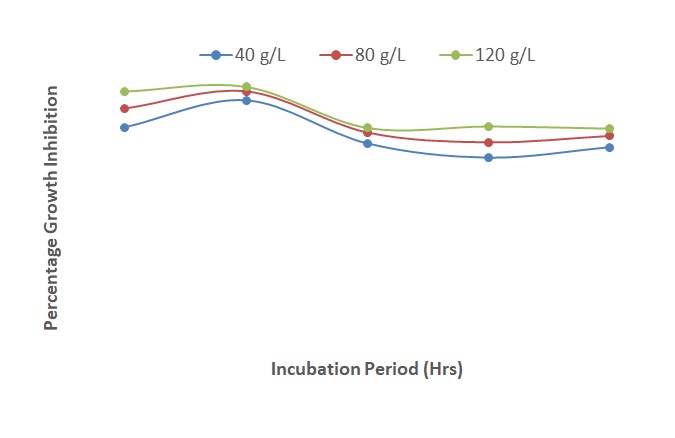 |
| Figure 8: Effect of interactions between different concentrations of extracts of Azadirachta indica and Zingiber officinale on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
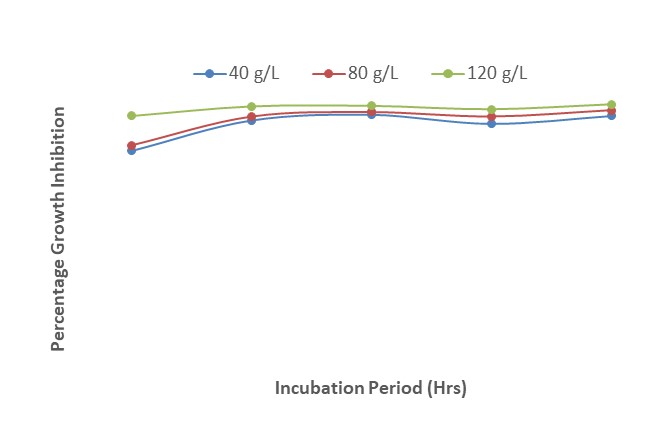 |
| Figure 9: Effect of interactions of different concentration of Azadirachta indica , Zingiber officinale and mancozeb on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
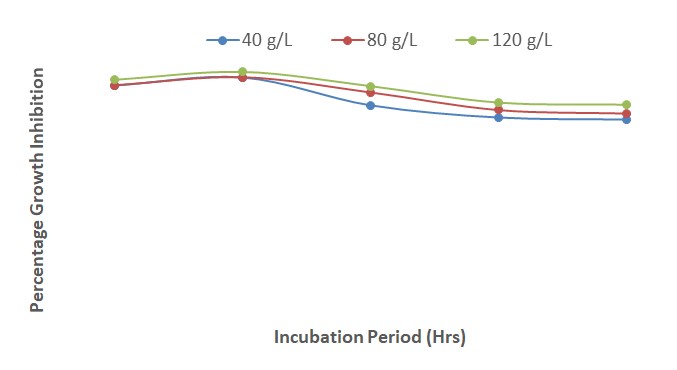 |
| Figure 10: Effect of concentrations of Piper guineense on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
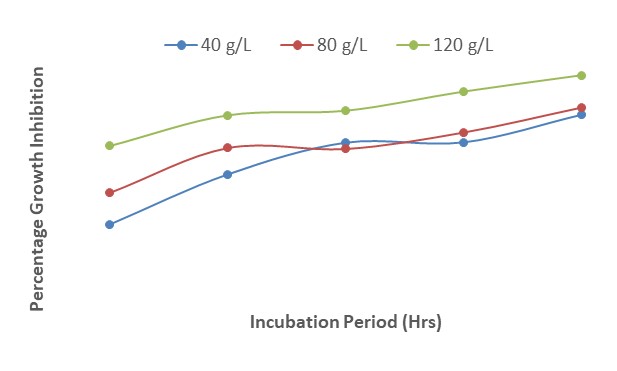 |
| Figure 11: Effect of interactions between different concentrations of P. guineense and mancozeb on the growth inhibition of Fusarium oxysporum after 120 hours of incubation |
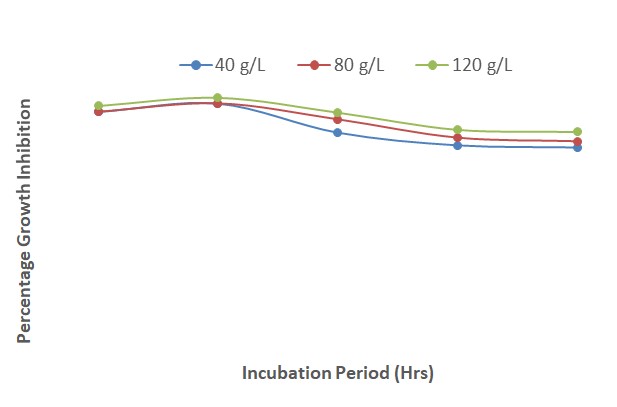 |
| Figure 12: Effect of interactions between different concentrations of Piper guineense and Zingiber officinale on the growth inhibition of Fusarium oxysporum after 120 hours of incubation |
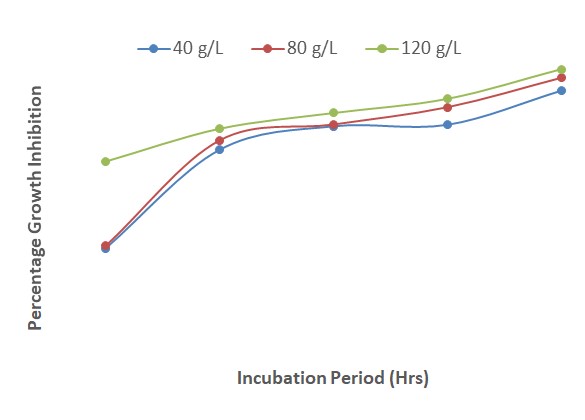 |
| Figure 13: Effect of interactions of different concentrations of Piper Guineense, Zingiber officinale and mancozeb on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
 |
| Figure 14: Effect of concentrations of Zingiber officinale on growth inhibition of Fusarium oxysporum after 120 hours of incubation |
 |
| Figure 15: Effect of interactions between different concentrations of Z. officinale and mancozeb on growth inhibition of F. oxyporum after 120 hours of incubation |
Tomato Part |
Frequency of isolates |
% frequency of isolates |
Root |
16 |
35.56 |
Fruit |
9 |
20.00 |
Stem |
12 |
26.67 |
Leaf |
8 |
17.77 |
Total |
45 |
100.00 |
Tomato part |
||
inoculated |
Uninoculated |
|
Root |
+++ |
- |
Fruit |
++ |
- |
Stem |
++ |
- |
Leaf |
++ |
- |
+ = slight rotting; ++ = moderate rotting; +++ = severe rotting; - = no rotting
Control Method |
|||||||||
40 g/L |
80 g/L |
120 g/L |
|||||||
Min |
Max |
Mean |
Min |
Max |
Mean |
Min |
Max |
Mean |
|
A. ind |
16.67 |
55.56 |
36.99±3.26i |
38.10 |
69.23 |
53.44±2.51i |
42.86 |
76.47 |
59.96±2.70i |
A. ind × Man |
69.23 |
89.86 |
84.48±1.36cde |
76.92 |
92.65 |
86.47±1.27cde |
76.92 |
92.75 |
88.73±1.17cd |
A. ind × P.guin |
52.38 |
82.35 |
64.55±2.60g |
56.25 |
83.33 |
67.35±2.35g |
62.07 |
88.24 |
71.11±2.21gh |
A. ind × P.guin × Man |
76.47 |
88.57 |
82.64±1.01de |
76.92 |
92.31 |
85.64±1.10de |
82.35 |
92.30 |
87.26±0.77de |
A. ind × P. guin × Z. offic |
73.81 |
88.57 |
80.33±1.09ef |
76.47 |
88.57 |
82.32±0.89e |
76.92 |
89.74 |
84.42±1.01e |
A. ind × Z. office |
57.14 |
84.62 |
67.51±2.01g |
57.14 |
87.18 |
71.53±2.38g |
66.67 |
88.24 |
74.56±1.79g |
A. ind × Z. offic × Man |
76.47 |
90.70 |
85.99±1.27cd |
76.92 |
76.92 |
87.77±1.27bcd |
84.61 |
94.87 |
91.86±0.71bc |
Mancozeb |
88.23 |
97.10 |
94.34±0.71a |
100.00 |
100.00 |
100.00±0.00a |
100.00 |
100.00 |
100.00±0.00a |
P. guin |
33.33 |
76.47 |
53.52±3.72h |
42.86 |
42.86 |
60.83±3.09h |
56.14 |
82.35 |
67.81±2.34h |
P. guin × Man |
76.92 |
92.75 |
87.98±1.13bcd |
82.35 |
82.35 |
89.08±0.71bcd |
84.61 |
95.58 |
92.20±0.80bc |
P. guin × Z. office |
66.18 |
89.74 |
75.43±1.83f |
69.12 |
69.12 |
77.27±1.40f |
73.68 |
89.74 |
79.56±1.45f |
P. guin × Z. office × Man |
82.35 |
95.83 |
91.67±1.02ab |
84.61 |
84.61 |
92.07±0.74b |
84.61 |
97.05 |
93.19±0.91b |
Z .offic |
52.94 |
82.86 |
66.69±2.41g |
63.16 |
63.16 |
72.07±1.99g |
63.24 |
84.62 |
73.79±1.74g |
Z. office × Man |
69.23 |
94.20 |
88.25±1.71bc |
76.92 |
76.92 |
90.83±1.17bc |
82.35 |
94.73 |
91.07±0.92bcd |
P-Value |
|
|
<0.01 |
|
|
<0.01 |
|
|
<0.01 |
A. ind = Azadirachta indica; Man = mancozeb; P. guin = Piper guineense; Z. office = Zingiber officinale; means on the same column with different superscripts are statistically significant (P<0.05)






































