Top Links
Journal of Pharmaceutics & Drug Development
ISSN: 2348-9782
Comparison of Physicochemical and Binding Properties of Novicel™ Microcrystalline Cellulose and Avicel® PH 101 in Direct Compression of Ascorbic Acid Tablets
Copyright: © 2023 Jimmy R Angupale. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Microcrystalline cellulose (MCC) is a key excipient in formulation and production of pharmaceutical solid dosage forms such as tablets. In this study, the microcrystalline cellulose (Novicel™) obtained from sugarcane bagasse (a waste material from sugarcane factories) was compared with Avicel PH 101 (a commercially available MCC) for its physicochemical characteristics and potential as a dry binder in direct compression of ascorbic acid tablets.
Keywords: Microcrystalline cellulose, Sugarcane bagasse, Direct Compression, Excipients, Ascorbic acid
Sugarcane bagasse (SCB) is a cellulose rich waste material generated by the sugarcane factories after extraction of the most valuable sugar pulp. With growing demand for sugar all over the world exceeding USD 52 billion in 2022 [1], the cultivation of sugarcane has multiplied triggering establishment or expansion of more processing industries. The global sugar cane production increased from 1.71 billion tonnes in 2008 to 1.87 billion tonnes in 2020 from cultivation of 20 million ha [1]. In East Africa, 36 million tonnes of sugar cane is grown annually generating the SCB waste of close to 4.86 tons [2]. Uganda, the leading East African country in the production of sugarcane produced over 514, 000 metric tonnes in 2019 and this is projected to reach 5.8 million metric tonnes by 2026 [3]. The increase in production of sugar cane is synonymous with generation of sugarcane bagasse waste which was reported to have reached 4,788 metric tonnes in 2021 in Uganda [4]. This abundant waste has been utilized mainly for generation of electricity, but in most sugarcane processing industries, only half of the waste is consumed for this purpose leaving excess to the environment [5].
The raw sugarcane bagasse contains 40 – 50 % of cellulose which has been exploited for development of various cellulose based materials including medical materials, packaging, absorbents, coating, and membrane production [6-7]. Cellulose is a semi-crystalline and water insoluble natural polymer consisting of D-glucopyranose units linked by β-(1-4)-glycosidic bonds. The amorphous and crystalline regions of the native cellulose can be separated using chemical methods (especially strong acids) which interfere with the bonds. The crystalline region can be further hydrolyzed into microcrystalline cellulose or even nanocrystalline cellulose for various pharmaceutical applications in drug delivery.
Microcrystalline cellulose (MCC), a pure partially depolymerized cellulose synthesized from α-cellulose precursor (crystalline region) is a white, odorless, tasteless powder composed of porous particles [8]. It was discovered in 1955 by Battista and Smith, and was first commercialized under the brand name Avicel [9]. MCC can be synthesized by various processes including reactive extrusion, enzyme mediated, steam explosion, and acid hydrolysis. The cellulose based material has been widely used in pharmaceutical industries as an excipient (a dry binder, disintegrant, absorbant, filler, lubricant, and anti-adherent) most commonly in tablet production due to its excellent compressibility and compactability [10]. The uniqueness of MCC to compress and consolidate efficiently in dry state makes it a vital and the most popular ingredient in production of pharmaceutical tablets using direct compression (DC) technology [9]. Direct compression is the cheapest and simplest method of tableting utilizing only two unit operations (mixing and compression) by-passing the traditionally used resource-intensive granulation process. DC is also convenient for drugs that are unstable in water due to hydrolysis such as ascorbic acid. MCC, when included in a tablet formulation, improves the mechanical integrity and physical characteristics of tablets which are of major importance in ensuring dosage stability during transportation and handling [11], thus making it an ideal diluent or binder for DC tableting method.
Despite the relevance and high demand of MCC in pharmaceutical industries and existence of methods to produce it from abundant waste material such as SCB, in Uganda and most other African countries, MCC and other pharmaceutical ingredients are still being imported [12]. Though the health sector in Uganda is said to depend on 80 % of imported health supplies most especially pharmaceuticals to sustain its needs, the 20 % medicines produced locally are still assembled from imported raw materials ranging from API (Active Pharmaceutical Ingredient) to excipients. This therefore necessitates the need to explore resources such as SCB for development of pharmaceutical grade ingredients especially at a time when the government is now advocating for value addition and industrialization to minimize dependence on imported goods. In an effort to contribute a solution to the dependence on imported MCC, the current work reports the comparison of critical material attributes (physicochemical parameters) and binding characteristics of Novicel ™, an MCC brand locally developed in Uganda from sugarcane bagasse waste material, to Avicel®, a commonly used commercial brand of MCC in most pharmaceutical industries in the country. Ascorbic acid was used as a model API for formulation of tablets by DC using Novicel™ and Avicel ® as dry binders and/or diluents.
Fresh sugarcane bagasse sample was obtained from Sugar Corporation of Uganda Ltd, SCOUL (Lugazi, Uganda); Hydrochloric acid, Sodium hydroxide, Potassium Iodide, Zinc Chloride, Ascorbic acid, Iodine, Hypochlorite used were of brand Loba Chemie Pvt. Ltd, (India); distilled water was obtained from Pharmaceutical Chemistry/ Analysis Laboratory of Mbarara University of Science and Technology (MUST); Avicel® PH 101), Stearic acid, Lactose, Silicon dioxide, and Talc were gifted by RENE Pharmaceutical Industries (Uganda).
The sugarcane bagasse sample was dried, milled in to a coarse powder (under #600 MIC sieve). Cellulose was extracted from the SCB powder and hydrolyzed into MCC using previously described methods with modifications [5, 13]. Briefly, the course powder was heated in sodium hydroxide (2.5 M) at 80℃ for 2 h for removing hemicellulose and lignin. Later, the mixture was filtered and the residue neutralized with excess distilled water. The residue was dried in oven at 80℃ and treated with 5.5% HNO3 to complete lignin removal and neutralized again using excess distilled water. The obtained cellulose was dried in an oven at 80℃ for 3 h.
The dried cellulose was bleached with 5% NaOCl at 80℃ for 2 hours, and finally hydrolyzed with HCl (2.5 M). The acidic mixture was filtered (Whatman No. 5) and thoroughly washed with distilled water until neutral pH. The residue was then dried in the oven at 80℃ for 2 hours. The final product (MCC) was milled and sifted through 150 MIC sieve, after which it was packaged into a plastic bottle and branded Novicel ™. The percentage yield of MCC obtained was calculated as:
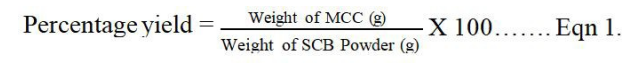
Physicochemical Characterization (Critical Material Attributes) of Novicel™ and Avicel® 101. Chemical identification. Chemical identification test for cellulose was performed using iodinated zinc chloride reaction test [14]. Iodinated zinc chloride solution was prepared by dissolving zinc chloride (20 g) and potassium iodide (6.5 g) in distilled water (10.5 mL). Then iodine (0.5 g) was added and the mixture shaken for 15 min. The sample (10 mg) was placed on to a glass slide and dispersed in 2 mL of iodinated zinc chloride solution. The change in color to a violet blue color was indicative of presence of cellulose Organoleptic characteristics. The color, odor, taste and physical appearance of MCC samples were examined and recorded in accordance with the pharmacopoeial specifications. For particle shape or morphological analysis, the samples (0.1 g each) were mounted on a glass slide followed by few drops of ethyl glycerol and levigation before placing a coverslip. The prepared slides of the samples were then viewed under a light microscope under magnification of X400 [15].
Moisture content. This was conducted using the automated moisture content analyzer (Metler,Toledo M133, Switzerland). The test samples (0.5 g each) were introduced onto the equipment sample holder, and the percentage of moisture measured according to established standard operating procedures of the analyzer. The procedure was repeated in triplicates for each sample. pH determination. A dispersion of 1 % was prepared for both samples using neutralized distilled water. The pH of the dispersions were measured using a calibrated pH meter (Elektro Genesis, Germany) and recorded. For each sample, the procedure was repeated in triplicates.
Powder Flow Properties. These included angle of repose, bulk density, tapped density, Carr’s index (CI), and Hausner’s ratio. They were determined according to methods previously described [16]: The angle of repose. This was determined using fixed height method. A glass funnel was clumped on a retord stand with its tip 10 cm above a flat surface. The samples (10 g each) were poured onto a funnel and allowed to flow freely to form a heap. The height and radius of the heap were measured and the procedure done in triplicates for each sample. The angle of repose was then calculated using the equation:
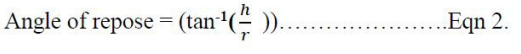
Where h - height of a heap and r - radius of a heap
Bulk Density (ρb). The samples (5 g each) were weighed and poured into a measuring cylinder (25 mL) tilted at an angle of 45 degrees to prevent tapping, and the volume recorded. The bulk density was then be calculated:

Where 𝜌b - bulk density, m - mass and v - volume.
Tapped Density (𝜌𝑡). The sample (5 g each) were weighed and poured into a measuring cylinder (25 mL), and tapped on a flat surface from a height (2 cm) until a constant volume obtained. The final volume was recorded and the tapped density calculated:

Where 𝜌𝑡- tapped density, m – mass, and v – final volume Carr’s Index (C.I). The Carr’s Index (%) of the sample was determined using the equation below:
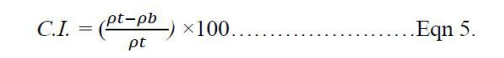
Hausner’s Ratio (H.R). This will be determined using the equation below:

Where “ρtapped” - Tapped density, and “ρb” - Bulk density
True Density and Porosity. A pycnometer bottle (25 mL) was filled with xylene up to its mark and the weight recorded. Some of the xylene was poured out and the sample (1.0 g) introduced inside the bottle. The bottle was topped with more xylene added up to the mark and wiped dry of excess fluid. The weight of the bottle was again recorded the second time. The true density (pt) and porosity were calculated using the following equations [15]:

Where; w is weight of the powder, a is weight of the bottle + solvent, b is weight of the bottle + solvent + powder, and SG is specific gravity of xylene
The porosity was calculated as:
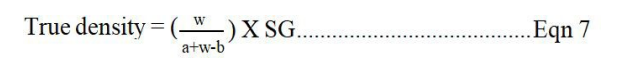
The samples (1 g) were placed in a 15 mL measuring cylinder and tapped. The tapped volume occupied by the sample was recorded as V1. Distilled water was then added up to the mark and the cylinder left undisturbed for 24 h. The new volume occupied by the sample was recorded as V2 and swelling index and capacity (%) calculated using equations below:
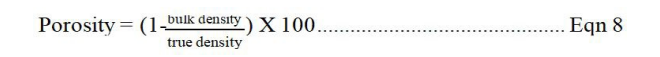
Where, V1 - initial volume of the sample, V2 - final volume of the sample
The samples (1g) were weighed into a dry silica crucible of known weight, then incinerated at 4500C in a muffle furnace for 6 hours until free from carbon. The crucible was then cooled, weighed and the percentage of the total ash calculated using the equation 10 [17].
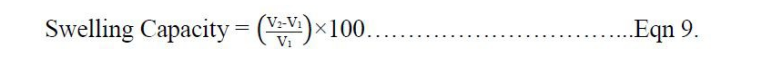
Where; X- weight of the empty crucible, Y - weight of the MCC sample taken, and Z - weight of the crucible + ash (after complete incineration)
The tablets were formulated using the formula shown in Table 1. The ingredients including MCC samples (Novicel™ and Avicel® 101), Lactose, Ascorbic acid, Stearic acid, Talc and Silicon dioxide were passed through a sieve (#150 MIC) to obtain powders with relatively uniform particle sizes, then weighed according to the formula in Table 1 for 50 tablets. MCC and Ascorbic acid were mixed together for 5 minutes and lactose added to it. Silicon dioxide and talc mixture was added to the sample mixed for another 5 minutes. To the mixture, stearic acid was then added and mixed for 5 min. The punch surfaces of the tablet press and die cavity was lubricated using a dispersion of stearic acid in ethanol (96%). After which, the powder mix was directly compressed using a single punch tableting press having flat surfaced punches of diameter 13 mm to make 50 tablets for each formulation.
A 1% stock solution of ascorbic acid was prepared and diluted with 0.1 M HCl to obtain five different concentrations in mg/mL (0.1, 0.2, 0.4, 0.6, 0.8, and 1) and the absorbance measured at 288 nm. A calibration curve of absorbance against concentration was plotted. Three tablets were randomly chosen and their average weight determined, after which they were crushed. A weight corresponding to the average was measured and dissolved in 0.1 M HCl (100 mL), their absorbance was then read using a UV/Visible spectrophotometer (6705 Jenway, England). The quantity of ascorbic acids and also the percentage content were calculated using the regression equation of the calibration curve.
Twenty (20) tablets were weighed individually and their average weight calculated. The percentage coefficient of tablet weight variation (% CV) was calculated for each brand sample according to the equation below [18]
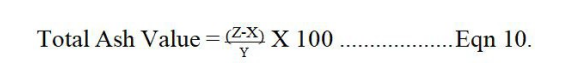
Six (6) tablets were de-dusted and collectively weighed (W1) then transferred to a friability test apparatus set to rotate at 25 r/min for 100 revolutions. The tablets were then removed from the friabilator (CS-I Laboao®, China), de-dusted and reweighed (W2) [19]. From the two weight values, the friability of each batch of tablets were calculated according to the equation 12:
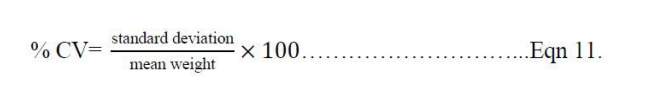
Ten (10) tablets were chosen randomly from each formula and their hardness measured using the hardness tester in KgF (VTHT-500, India).
Six (6) tablets randomly selected from each of the formulas were placed in separate tubes in the basket rack assembly of a tablet disintegration test apparatus. 0.1M HCl medium was maintained at a temperature of (37± 0.5) 0C. The disintegration tester (DTG 2000 COPLEY, UK) was then started and the tablets carefully observed until the time (minutes) when no residue remained on the mesh screen. The apparatus was then stopped and time recorded [20].
The paddle dissolution test apparatus was employed in which 900 mL of 0.1 M HCl dissolution media was maintained at (37.0 ± 0.5) 0C with a rotation speed of 75 r/ mins. The dissolution test was performed for five tablets for each batch or formula and 5mL of the sample was drawn with replacement using the same media kept at the same temperature at time intervals of 5, 10, 15, 30, 45 and 60 min. The absorbance of the drawn samples were read at maximum wavelength of 288 nm. The ascorbic acid concentration was calculated from the standard plot equation and its amount in the samples determined using the equation below:
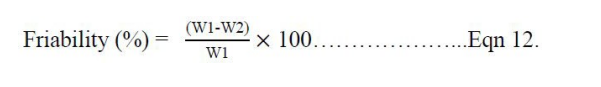
Percentage of drug released was calculated using the equation below

A graph of cumulative percentage release of ascorbic acid against time (mins) was plotted to represent the dissolution profile of tablets made using the two samples of MCC as binder-diluent. A model independent approach as recommended by US FDA guidance for dissolution data equivalence involving use of similarity factor (f2) and difference factor (f1) was adopted for comparing the dissolution profiles of test sample Novicel™ and Avicel® as the reference [21]. The two factors (f1 and f2) were calculated using the following equations:
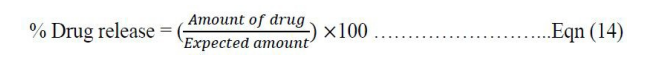
Where n is the number of time points, Rt is the dissolution value of the reference batch at time t, and Tt is the dissolution value of the test batch at time t.
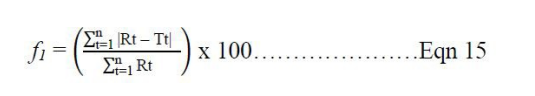
The data was summarized into means ± standard deviation (Mean ± SD) and presented in suitable tables and graphs. Statistical analysis was done using student’s t-test to detect any significant differences between the two groups. Significant mean differences were considered at P ≤ 0.05. The GraphPad prism software (version 8.0.2. 263) was used for data analysis.
The percentage yield of of MCC obtained from Sugarcane Bagasse (SCB) was 34.43 %. Both Novicel™ and Avicel® 101 showed positive test with iodinated Zinc Chloride (color turned to violet blue), thus confirming the presence of cellulose in the MCC. The MCC samples were both odorless, tasteless, and white in color (as shown in Fig 1). The powder samples also had irregularly shaped (rod shaped) particles intertwined with aggregates of a few spherical particles as shown in Fig. 2. Morphologically, the Novicel® had longer particles compared to the Avicel® based on the microscopic images at the same wavelength. The moisture content of Novicel™ was significantly higher than the one of Avicel® 101 as shown in Table 2. Novicel™ had a pH of 6.25 ± 0.5 comparable to that 6.45 ± 0.4 of Avicel® 101 as shown in Table 2. Despite the differences, all the values of moisture content and pH were in the acceptable pharmacopoeial ranges for MCC pharmaceutical grade.
Regarding the comparison of the powder flow properties of the two samples, their angle of repose were all above 42o and statistically similar at P≤ 0.05 as shown in Table 2. Avicel® 101 had significantly higher bulk and tapped densities compared to Novicel® sample indicating better packing characteristics. The Carr’s Indices (CI) and Hausner’s Ratios (HR) of the two MCC samples were comparable all standing at 38 % and 1.6 respectively (Table 2). The Novicel® MCC demonstrated significantly higher true density and porosity compared to the Avicel standard implying that, the latter is better in terms of compaction and packing characteristics which is in agreement with the particle shape, bulk and tapped densities described earlier. Similarly, Avicel® 101 also showed significantly higher swelling power or capacity compared to Novicel™ at P≤ 0.05 (Table 2), and thus expected to have faster disintegration. Also the total ash value of Novicel® is significantly higher than the one of Avicel™ indicating lesser levels of metallic components in the standard commercial brand (Table 2).
The ascorbic acid tablets (200 mg) were formulated using the two MCC samples (Novicel™ and Avicel®) as dry diluent-binders and powder mix directly compressed. A calibration plot of ascorbic acid for assay of the tablets was obtained (Fig.3) with a linear regression equation of Y = 0.9422X - 0.0713 (Where Y-Absorbance and X- concentration in mg/ml), and a correlation coefficient (r2) of 0.966. Both formulations had tablets with ascorbic acid content in the range of 99 – 102 % with Avicel® tablets showing significantly higher assay, but all of them lied in the acceptable ranges (Table 3). Hardness of both brands were similar and all lying within the pharmacopoeial requirements (Table 3). The tablets for both brands all had friability percentages from 0.2 – 0.5 % (Table 3) with Novicel™ tablets having significantly lower values compared to Avicel® 101, even though all had values less than 1 % (the acceptable limit). The tablets of Novicel™ disintegrated significantly faster than those of Avicel® 101 (Table 3), but both complied with the international standards.
The dissolution profile of the ascorbic tablets formulated using the two brands shows that both released over 80 % of the active ingredient (ascorbic acid) within 15 min (Fig 4). Both curves appeared superimposable implying that the two brands of MCC may possess similar drug release characteristics. The difference or dissimilarity and similarity factors obtained (Table 3) also indicated that the two curves are similar, thereby confirming that the two tablet formulations using Novicel™ and Avicel® as filler-binders are equivalent.
MCC yield obtained in our study was similar to the one of [5] in India, but lower compared to 50 % reported recently in Ethiopia [22]. This variation could be attributed to differences in the cellulose extraction methods adopted by the studies. The latter used H2O2 to remove hemicellulose and lignin while the studies with 30 % yield used nitric acid. It is possible the HNO3 may be more efficient in removing extraneous matters such as lignin and hemicellulose compared to H2O2 leading to false higher yields. Differences in geographical locations might also affect the quantity of pure cellulose in the SCB from different parts. Various sources have reported that 40 – 50 % of SCB total mass is cellulose [23,6,7, 22], therefore, the lower yields could just be as a result of losses incurred during the handling and processing steps during isolation of cellulose and its hydrolysis into MCC.
The identification tests, pH, moisture content, and organoleptic properties of both Novicel™ and Avicel® obtained in our study all lie in the acceptable international or pharmacopoeial limits for pharmaceutical grade MCC [24, 8] – pH 5 – 7.5; moisture content ≤ 5 – 7; odorless; white in color. Even though, there are statistically significant differences especially in pH and moisture content values of the two brands, the critical material attributes or specifications already established override. This means, they all meet quality requirements for pharmaceutical use
The angle of repose of Avicel® and Novicel™ were statistically similar and all lie within the ranges of passable and poor respectively according to [25] classification – angle of repose < 30o shows excellent flow, 31 – 35o means good flow, 36 - 40o indicate fair flow, while 41 - 45o refer to a passable flow with a need for a flow aid, and 46 - 55o indicate poor flow. Generally MCC is known to have poor flow characteristics mainly originating from its rod shaped particles as shown in Fig. 2 and other previous studies [9]. Therefore, the need for a flow aid in a formulation utilizing MCC as direct compression diluent and dry binder is highly recommended.
Bulk density is an indicator of the powder’s packing characteristics and its ability to undergo compression (higher values are desirable). It depends primarily on particle size distribution, particle shape and the tendency of the particles to adhere to one another [26]. The higher bulk and tapped densities of Avicel® compared to Novicel™ could have resulted from the larger particle size and more rod shape of the latter influencing it to have poor packing with higher porosity thus lowering the bulk and tapped densities. Despite the differences between the two brands, the values all lied within the acceptable limits of British Pharmacopoeia for MCC [8] Carr’s index (CI) and Hausner’s ratio (HR) are important indices in describing powder flow properties. Both brands showed statistically similar values and all parameters lying beyond the range of very poor according to USP classifications [8]: 32 – 37 and 1.46 – 1.49 for CI and HR respectively. These concur with the angle of repose findings discussed earlier, further conforming to the known poor flow properties of MCC.
The true densities for the two brands (1.4) were slightly lower than 1.5 reported in a previous study for MCC [27]. Lower true densities are desirable requiring lower pressure for powder compactability and consolidation during tableting. Avicel® had statistically lower values compared to Novicel™, therefore the standard MCC would be expected to compress more easily than Novicel™ at the same pressure. Conversely, a higher true density also leads to high compact density in direct compression and small sized tablet formation which facilitates swallowing [28]. Therefore Novicel™ would be expected to have high compact density and form smaller tablets compared to Avicel®. The significantly higher porosity obtained for Novicel™ compared to Avicel® agrees with the poor packing characteristics indicated earlier by bulk and tapped densities.
Swelling index is the increase in volume of a sample after water absorption and higher swelling is a key of good disintegration ability for MCC [29]. The higher the swelling index of a powder, the better the water penetration which allows good disintegration of tablets. The swelling index of Avicel® and Novicel™ are statistically comparable, which indicates that they could be having similar disintegration properties.
Total ash values indicates the level of inorganic substances present in the samples and also the safety of the MCC. According to USP [8], the maximum total ash cannot be more than 20% which is over 60 times higher than the values obtained in our study for both brands. Though Avicel® had significantly lower values compared to Novicel™, both have extremely low levels and comply with the standard requirements for MCC.
Assay analysis checks for the ability of the tablet batch to possess the required amount of the active ingredient. According to the USP [30], the amount of drug in each tablet should lie in the range of 85% - 115%. Both brands had assay percentages within the range (99 – 102 %), thus demonstrating acceptable contents.
Weight uniformity is a compendia requirement for high dose tablet dosage forms (drug content ≥30 %). The tests for weight uniformity for both brands complied with the BP specifications i.e. tablets with an average weight less than and greater than 3g should have a variation of ±7.5 and ±5 respectively [31]. Our tablets with average weight of 0.5 g had coefficient of variations ≤ 5, hence meeting the requirement for weight distribution and expected to also have acceptable content uniformity.
The hardness values of tablets from both brands were statistically similar and all within the acceptable range (4 – 10 KgF) [24], though Novicel™ showed slightly higher tablet crushing strength. This could be due to the higher moisture content as discussed earlier for Novicel™, higher moisture levels in MCC are known to enhance consolidation, increase bonding especially solid-bridges that are useful in direct compression, resulting into stronger tablets [27]
Friability indicates the strength of tablets and its resistance to abrasion during normal handling, packaging and transport. The friability of Avicel® tablets were significantly higher than ones of Novicel™ which corresponds to the trends of hardness reported in our study i.e. harder tablets having lower friability. Though Avicel® tablets had higher friability, both tablets formulated from the respective brands exhibited friability less than 1%, thus complying with the compendial requirements [32].
The disintegration test is a preliminary test to predict the release of the drug from the medicine (dosage form). According to BP, a well formulated and manufactured immediate release tablet dosage form should disintegrate within 15 min while the USP specifies 30 min. Based on these pharmacopoeial requirements [20], all the brands under investigation passed the test for disintegration and are expected to readily release the drug. Tablets from both brands of MCC disintegrated in less than 3 min, meaning they can be used even in formulation of fast disintegrating tablets or related solid dosage forms
Dissolution is a critical or an official test parameter that predicts the ability of a tablet formulation to release the active ingredient before absorption to elicit its intended therapeutic activity. According to British Pharmacopeia, tablets that release more than 85% of active ingredient within 30 min are regarded as rapid releasing [33]. USP also indicates that immediate release tablets are expected to release over 70 % of its active drug within 45 min [30]. Cumulative percentage release for tablets formulated using both Novicel™ and Avicel® had over 80 % of ascorbic acid in solution after 15 min, meaning they all passed the requirements for immediate release and would be absorbed if administered orally. In comparison of the dissolution profiles of tablets from the two brands, difference or dissimilarity factor, f1 and similarity factor, f2 calculated for Novicel™ in reference to Avicel® tablets were all in the acceptable criteria for similarity (Table 3) [34]. This means that both brands of MCC influence the release of ascorbic acid in the same way, and their tablets can be considered to be pharmaceutically equivalent.
Considering the physicochemical properties of Novicel™ and its dry binding properties demonstrated in Ascorbic acid tablet formulation as compared to Avicel®, the MCC has the potential to be used as disintegrant, filler, binder, absorbant, and even antiadherant in formulation of other solid pharmaceutical dosage forms [27, 9]. These are all useful functions in preparations of capsule, pellet, powder, and even granule formulations. In semi-solid and liquid pharmaceutical formulations, it could also be used as thickener or viscosity enhancer in suspensions and gels, and even in food industries [8].
Utilization of sugarcane bagasse waste for production of Novicel™ MCC in Uganda will contribute immensely in reduction of biomass burden of the waste and its associated undesirable impacts to the environment. As much the sugarcane factories are devising means to consume the waste for generation of energy (electricity) and bioethanol, only half of total bagasse produced is utilized, leaving an excess to the environment. In developing countries such as Uganda, accumulation of organic wastes including bagasse is a major problem as most are disposed off into landfills which become breeding grounds for insects, parasites and many other pathogens [35], thus threatening public health and the environment in general. This has been reported in Sudan, where lack of proper waste management system in sugarcane factories led to increase in parasite multiplication, impaired crop and animal production, increased cases of eye and respiratory diseases resulting from suspended particles and bagasse flies [36]. Therefore, the conversion of sugarcane bagasse into Novicel™ MCC is a novel approach to waste management and environmental protection in Uganda, in addition to value addition to the waste material and import substitution of MCC for local pharmaceutical industries.
The Novicel™ conforms to the required critical quality attributes or physicochemical parameters of pharmaceutical grade MCC comparable to the standard Avicel® PH 101. The tablet formulations made using both brands all met the pharmacopoeial specifications for immediate release tablets while exhibiting similar drug release characteristics, thus making them pharmaceutically equivalent in vitro. Therefore, Novicel™ is a potential filler – dry binder microcrystalline cellulose that can work as same as Avicel® PH 101 in direct compression tableting.
We are very grateful to the Government of Uganda through Directorate of Research and Postgraduate Training (DRGT), Mbarara University of Science and Technology for the funding (Grant No: DRGT/SG/FY22-23/R2/T2P20) that facilitated the activities of this work. We also thank our research assistants Ms. Madinah Natenge and Mr. Nuwamanya Alex for their enormous help during the implementation of different research activities.
 |
| Figure 1: : Comparison in color of Novicel (MCC SC Bagasse) and Avicel PH-101 (MCC RENE, sample powder of Avicel PH-101 obtained from RENE Pharmaceutical Industries Uganda). |
 |
| Figure 2: Light microscopic images of Novicel (SCB x400) and Avicel 101 (Avicel x400) |
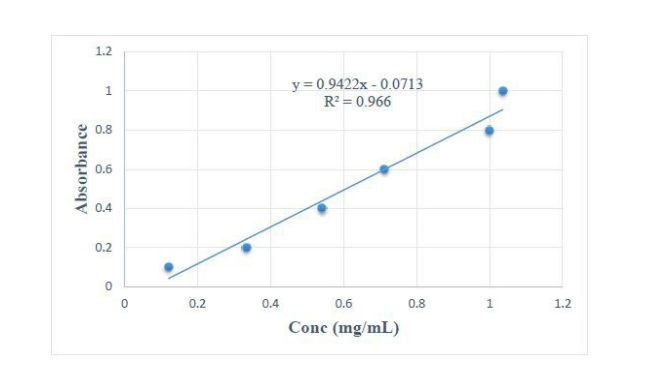 |
| Figure 3: Calibration plot for ascorbic acid |
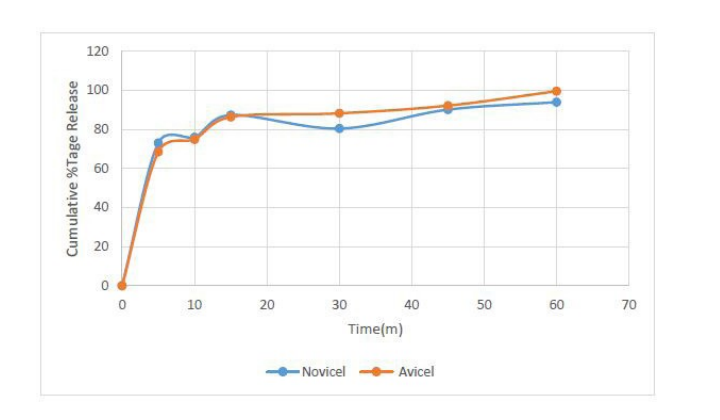 |
| Figure 4: Dissolution Profile of Ascorbic acid Tablets |
Ingredients (mg) |
F1 (Novicel™) |
F2(Avicel®) |
Ascorbic acid |
200 |
200 |
Novicel™ |
250 |
- |
Avicel® 101 |
- |
250 |
Lactose |
44 |
44 |
Stearic acid |
1.0 |
1.0 |
Talc |
2.5 |
2.5 |
Silicon dioxide |
2.5 |
2.5 |
Total (mg) |
500 |
500 |
Parameters |
Avicel® 101 (Mean ± SD) |
Novicel™ (Mean ± SD) |
P-value (P≤0.05) |
Moisture content (%) |
4.337 ± 0.219* |
5.370 ± 0.220* |
0.0045 |
pH |
6.45 ± 0.050* |
6.25 ± 0.050* |
0.0080 |
Angle of repose (°) |
42.85 ± 3.219 |
48.13 ± 0.767 |
0.0507 |
Bulk density (g/mL) |
0.4004 ± 0.016 * |
0.2609 ± 0.004* |
0.0001 |
Tapped density (g/mL) |
0.6668 ± 0.000* |
0.4227 ± 0.010* |
< 0.0001 |
Carr’s index (%) |
39.94 ± 2.408 |
38.24 ± 2.421 |
0.4364 |
Hausner’s ratio |
1.667 ± 0.067 |
1.621 ± 0.065 |
0.4429 |
True density (g/mL) |
1.412 ± 0.006* |
1.466 ± 0.004* |
0.0002 |
Porosity (%) |
71.64 ± 1.107* |
82.20 ± 0.304 * |
< 0.0001 |
Swelling capacity (%) |
60.04 ± 0.057 |
40.03 ± 0.053 |
< 0.0001 |
Ash value (%) |
0.2067 ± 0.025* |
0.3267 ± 0.032* |
0.0070 |
Parameter |
Avicel® 101 (Mean ± SD) |
Novicel™ (Mean ± SD) |
P-value |
Assay (%) |
102.0 ± 0.5307* |
99.30 ± 1.061* |
0.0179 |
Weight uniformity |
0.5125 ± 4.278 |
0.5031 ± 5.505 |
0.2437 |
Hardness (KgF) |
9.630 ± 0.538 |
10.25 ± 0.185 |
0.1307 |
Friability (%) |
0.5778 ± 0.183* |
0.2299 ± 0.027* |
0.0312 |
Disintegration (s) |
83.33 ± 11.02* |
43.33 ± 7.572 * |
0.0066 |
Dissolutionequivalence |
Factors |
Similarity criteria |
|
|
f1 |
4.315 |
0 – 15 |
|
f2 |
67.050 |
50 – 100 |






































