Top Links
Journal of Nursing and Patient Health Care
ISSN: 2767-9292
Prevalence of Significant Ocular Surface Symptoms and Its Relation to Polypharmacy Among In-Patients in A General Internal Medicine Department
Copyright: © 2022 Jonsdottir F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Purpose:The aim was to estimate the prevalence of significant ocular surface symptoms among patients admitted to a general internal medicine ward and to evaluate the relationship between dry eye syndrome and sex, age and polypharmacy
Methods:The prospective, descriptive study conducted included patients aged 18–85 years admitted between December 2019 and March 2020. The patients answered the Ocular Surface Disease Index (OSDI), a standardized questionnaire about dry eye symptoms. In addition, tear secretion was measured by the Schirmer-I test. Standard data on treatment were retrieved from the patients' medical records.
Results:One hundred patients (53% female) were recruited from a general internal medicine ward in a secondary teaching hospital in Iceland. Twelve patients were excluded due to incomplete Schirmer-I tests. The average age of the included patients was 66 years (22–85). Of the 88 participants, 51.2% experienced dry eye symptoms according to the OSDI questionnaire, and more than half (57.9%) had abnormal Schirmer-I tests. The prevalence of significant ocular surface symptoms was higher among female patients (60.4% vs 55%) and elderly patients (≥50 years). The prevalence of significant ocular surface symptoms was higher among patients on polypharmacy treatment (61% vs 36.4%).
Conclusion:The prevalence of significant ocular surface symptoms in an internal medicine ward is high. Patients on polypharmacy treatment, elderly and female sex are more likely to experience symptoms of ocular dryness.
Keywords: Dry Eye Syndrome, Drug-Induced Sicca Syndrome, Polypharmacy, Prevalence, Keratoconjunctivitis Sicca
Subjective complaints of dry eye syndrome (DES) are common, especially among older people [1]. DES causes ocular irritation and affects ocular health and visual performance [2,3]. Patients with mild to severe dry eye experience reduced quality of life at a level similar to that experienced by patients with moderate to severe angina pectoris [4].
According to the Tear Film and Ocular Surface (TFOS) Society's Dry Eye Workshop (DEWS) II report, dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film. It is accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles [5].
The prevalence of DES ranges from 5–50% worldwide. DES is more frequent among females, and its prevalence increases with age [6,7]. The prevalence of sicca symptoms is currently 20.3% in Iceland [8].
A variety of conditions underly DES. It can occur due to inflammatory diseases (e.g., Sjogren's syndrome), hormonal imbalance (e.g., in perimenopausal women), environmental conditions (e.g., dry climate) and the use of contact lenses [9,10]. Meibomian gland dysfunction can also result in tear film instability [5,11]. Furthermore, systemic medications can cause DES, such as anticholinergic medication, secondary to decreased tear production, altered nerve input and reflex secretion, inflammatory effects on secretory glands or direct irritation effects through secretion into the tears [12,13]. Topical drugs and additives, including preservatives such as benzalkonium chloride, can alter the homeostasis of the tear film and ocular surface, thereby inducing dry eye symptoms [10,14]. Few studies have evaluated the influence of systemic use of medication on the prevalence of DES in selected age groups [15,16]. Most of these studies used only questionnaires concerning dry eye symptoms rather than objective tests to measure aqueous tear production.
The similarity between saliva and tear secretions is high, as the automatic nervous system and its nerves are very similar. Thus, their mechanisms of action are similar. Studies suggest that polypharmacy therapy is the leading cause of dry mouth, with a prevalence of up to 82%. However, polypharmacy therapy has not been studied as a cause of dry eye [15].
The frequently used definition of polypharmacy therapy is five or more medications [17], which is most commonly seen in the older patients [18,19]. Medication side effects are three times more common in the older patients, which is the age group that suffers most from symptoms of dry eye [15].
In the studies mentioned above, no information was available on the prevalence of dry eye symptoms in hospitalized patients, for example, in a general internal medicine ward. This prospective analysis aimed to estimate the prevalence and disease burden of DES and the effect of polypharmacy by using objective and patient-reported symptoms in a hospitalized patient group.
This prospective descriptive study included individuals aged 18–85 years admitted consecutively to a general internal medicine ward at Landspitali, the National University Hospital in Iceland, from 5 December 2019 to 31 March 2020.
Data on medication history were collected from the medical records and the hospital prescribing e-system, "Therapy®", where all medications and medicinal forms listed independent of administration.
Participation involved answering a standardized questionnaire about dry eye symptoms, the Ocular Surface Disease Index (OSDI) questionnaire. The results from the OSDI were classified into four categories with respect to the severity of dry eye symptoms: 1) normal (0–12 points), 2) mild (13–22 points), 3) moderate (23–32 points) and 4) severe (33–100 points) [20]. See the supplementary file.
Secondly, the patients underwent a Schirmer-I test (performed without topical anaesthesia). Standardized filter paper strips (Entod Research Cell, Tottenham, London) were put over the lower lid's lateral third. After five minutes, the filter paper was removed, and the length of the strip wetted by tears was measured [21]. An abnormal Schirmer-I test is defined as less than 5 mm of moisture on the filter paper in five minutes. If the measurement from one or both eyes was less than 5 mm, it was considered DES [22]. No artificial tears or other eye drops had been used for at least 30 minutes prior to performing the Schirmer-I test.
Patients who did not speak Icelandic, or in isolation due to their medical condition, patients with cognitive impairment and those whose caregivers considered them incapable of participating were excluded from the study.
A patient consent form was obtained from all participants. The Ethics Committee of the hospital and Data Protective Authorities approved the study protocol (License number: 33/2019).
Statistical analyses were performed by statistical software R. Studio 1.2.5033. Descriptive statistics used to describe the population were means, standard deviations and rates. Linear logistic regression was used to examine the relationship between certain variables. The results of the logistic regression were presented with a p-value. Statistical comparisons were made with a t-test. Statistical significance was assessed with a 95% confidence interval and a p-value lower than 0.05.
Of the 247 participants who participated in the study, 100 fulfilled the inclusion criteria. Of those 100, 12 patients had incomplete Schirmer-I tests. The reason for an incomplete Schirmer-I test was in the case of patients who requested that the test be stopped before five minutes had elapsed. Thus, 100 individuals were included in the final analysis regarding the OSDI questionnaire, and 88 individuals were included in the analysis regarding the outcomes of the Schirmer-I test. Supplementary file 1 presents a flow diagram demonstrating the composition of our study population. Table 1 shows the demographic data of the participants.
According to the OSDI questionnaire, 18% of patients reported moderate symptoms and 11% severe significant ocular surface symptoms. Meanwhile, 51% of patients had abnormal Schirmer-I tests (< 5 mm/5 min). Of patients who considered themselves to have severe significant ocular surface symptoms, 63.6% had an abnormal Schirmer-I test (Table 2). The logistic regression analysis results showed an r2 value of 0.012 and a p-value of 0.31. Therefore, it is impossible to estimate a relationship between the variables of the Schirmer-I test and the OSDI questionnaire where p>0.05.
When prevalence was examined between the sexes, 17% of men and 20.8% of females experienced moderate ocular surface symptoms, according to the OSDI questionnaire. Females also experienced more severe symptoms of dry eye (17%) compared to men (2.2%) (Figure 1). Proportionally, more women (60.4%) had an abnormal Schirmer-I test compared to men (55%). Of the 12 excluded patients due to incomplete Schirmer-I tests, 58.3% were men.
In the age group of 18–49 years, 14.3% experienced moderate ocular surface symptoms compared to 19.8% in the 50–85 age group (Figure 2). Proportionally, more individuals in the 50–85 age group had an abnormal Schirmer-I test compared to the 18–49 age group. Of the 12 patients excluded from an incomplete Schirmer-I test, 83.3% were 50–85 years old (Figure 3).
When the severity of symptoms of dry eye in patients receiving polypharmacy therapy (i.e., more than five medications) was examined, the results showed that 19.8% of patients experienced moderate ocular surface symptoms and 12.7% severe ocular surface symptoms of dry eye versus those who were not on polypharmacy therapy - 7.1% and 0%, respectively. Of patients on polypharmacy therapy, 61% had an abnormal Schirmer-I test compared to 36.4% of patients taking less than five medicines (Table 3). Most patients (n = 74, 84.1%) were taking the ATC code N - Nervous system medicine; of those, 56.8% had abnormal Schirmer-I tests. Only three patients reported using artificial tears regularly
In the present study, we estimated the prevalence of significant ocular surface symptoms in consecutive patients attending an acute medicine department due to various medical conditions. We used the Ocular Surface Disease Index (OSDI) and objectively measured the aqueous tear production with the Schirmer-I test. In the study, we aimed to evaluate the in-patients bedside and, for that reason, choose the Schirmer test even though the certainty of the test is not perfect. However, we decided to use the Schirmer test as it was suitable for the study setting.s. It would have been interesting to do other tests, for example, tear breakup time or tear osmolarity test, but the setting in the ward did not offer it as we did not want to transfer in-patients. To our knowledge, this is the first study focusing on symptoms of ocular surface symptoms in a hospital population. Nearly half our patients reported ocular surface symptoms according to the OSDI questionnaire, of whom 29.6% had moderate or severe symptoms, and nearly two-thirds also had an abnormal Schirmer-I test. A higher proportion of patients with polypharmacy had higher OSDI scores and abnormal Schirmer test results than those without polypharmacy therapy. Only three patients reported using artificial tears regularly may indicate a lack of knowledge of dry eye or that symptoms are not severe enough to seek treatment. It should also be taken into account that artificial tears in Iceland are not reimbursed unless the patient has confirmed a diagnosis of Sjogren's Syndrome. The objective results of the OSDI questionnaire and Schirmer's test remain. Thus, DES should be considered in the routine care of patients in a general medicine hospital ward.
Studies on the prevalence of DES among healthy individuals have been conducted in the United States [16], Singapore [23] and Denmark [24]. In these studies, the prevalence of DES increased with age and was higher among women. The results of the studies mentioned above were comparable to those in the present study. It is not possible to claim that the prevalence of significant ocular surface symptoms among our female patients is higher only due to polypharmacy. It may also relate to sex and hormonal imbalances. However, in this context, it is worth mentioning that these studies had a higher number of participants, and their results were based solely on self-reporting questionnaires to estimate the prevalence of sicca symptoms. In contrast, we used a structured interview based on OSDI, and we also objectively measured aqueous tear production rates with the Schirmer-I test. The Danish study is comparable in that respect; as the incidence increases with age, however, the comparison must be made with caution, as the oldest participants in the Danish study were 60 years of age, compared to 85 years in the present study, and our study population comprised individuals in need of a medical ward.
In Iceland, Atladóttir et al. (2000) studied the prevalence of dry eye and mouth symptoms regarding Sjogren's syndrome. Their study included two age groups, 40–49 years and 70–75 years, and used a questionnaire sent to a random sample of the Icelandic population in the Reykjavik area. The results showed that 20% of individuals experienced symptoms of dry eye. Of 621 participants, 23 who had all three main symptoms of Sjogren's syndrome — sicca symptom, fatigue and joint pain — were invited to further examination with the Schirmer-I test. The results showed that 26% of individuals reporting this triad of symptoms had an abnormal Schirmer-I test and that women were more likely to experience dry eye symptoms. Atladottir et al. (2000) studied the prevalence of primary Sjogren's syndrome in Iceland, which was calculated to be 0.2% with a confidence interval of 0.0–0.5%. The difference between the prevalence of dry eye can be explained by the fact that Atladottir et al.'s study was a randomized population-based study compared to our study, which was conducted in hospital surroundings.
The current study's main strength is based on an international and well-standardized questionnaire (OSDI) and included an objective test (i.e. the Schirmer-I test) to evaluate tear production. This study is also a prevalence study and therefore provides information on the frequency and symptoms of significant ocular surface symptoms among in-patients in a general medicine ward. A single investigator (HRS) performed all interviews and Schirmer-I tests in the present study after receiving training from an ophthalmologist (GMZ). Using only one investigator reduces the likelihood of discrepancies between tests and diminishes inter-observer variability. The OSDI questionnaire is accepted as a screening tool for dry eye disease [12]. Of importance, the study aimed to examine the potential effects of polypharmacy on tear production and dry eye. Therefore, the Schirmer's test was chosen as a diagnostic test. Meanwhile, the workup did not include markers of homeostasis on the ocular surface, i.e. tear breakup time, osmolarity or surface staining.
The study's main limitation is that the study population is relatively small, as it was carried out in only one hospital ward. Most patients who participated in the study were elderly individuals — more than half were aged 70 years and older. This may have given us a higher prevalence than in other specialist wards, as dry eye symptoms are known to increase with age (Messmer, 2015). Another key limitation for the study is that the study population was carried out in sick in-patients where polypharmacy is common. For that reason, the results need to be interpreted with caution. Further, this study was conducted over four winter months. Therefore, it is possible that the prevalence of ocular surface symptoms would have been different if this study had covered a more extended period or a different time of year [25]. The humidity was measured, and the average humidity of the ward was 23.4% HR. The humidity in Iceland is around 25-40% HR during the winter months when we conducted our study. When the humidity is low, it can cause the mucous membranes to dry out, and people can feel discomfort in their eyes. However, this needs to be interpreted with caution as it is unknown whether the ward’s ward's air humidity or thepatient's general environment caused dry eye symptoms. It should be emphasized that in the OSDI interview, patients are asked to recall the frequency of ocular symptoms and scenarios experienced in the past week. The interview was conducted on the first or second day after admission. Other conditions such as allergy and pollution were not examined.
Our study demonstrates a high prevalence of significant ocular surface symptoms in a general internal medicine ward. The majority of the patients in the present study were seriously ill and on polypharmacy therapy. The results also indicate that the prevalence of significant ocular surface symptoms is higher among women and older individuals, and many of them had moderate or severe dry eye symptoms. This study suggests that significant ocular surface symptoms may be a common side effect of medical treatment. Healthcare professionals can reduce the burden of this side effect, for example, by reviewing their medical treatment and providing information and artificial tears during the hospital stay.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
The author(s) received no financial support for the research, authorship and/or publication of this article
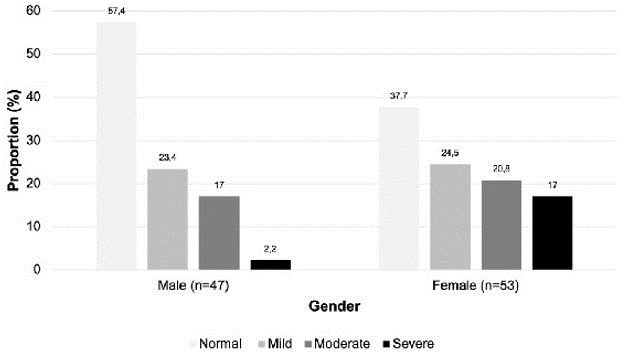 |
| Figure 1: Proportion of patients with symptoms of dry eye according to the Ocular Surface Disease Index (OSDI) questionnaire classified as normal, mild, moderate or severe and by gender |
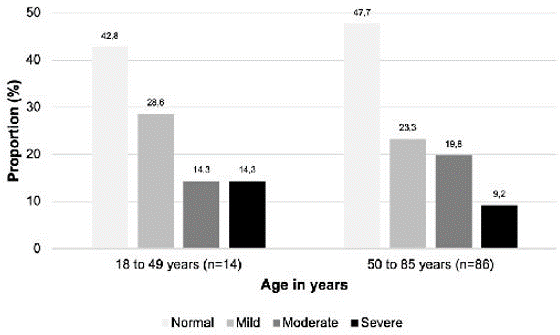 |
| Figure 2: Proportion of patients with symptoms of dry eye according to the classification by the OSDI questionnaire (Ocular Surface Disease Index), classified in two age groups |
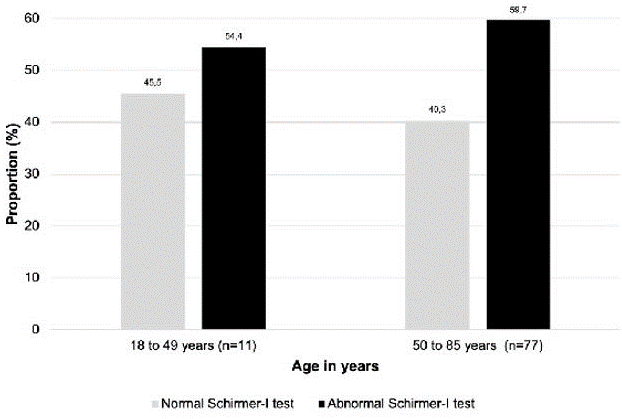 |
| Figure 3: Proportion of patients with normal and abnormal Schirmer-I test, classified by age groups |
|
OSDI questionnaire |
Schirmer-I test |
Number (%) |
|
|
Male (%) |
47 (47.0) |
40 (45.5) |
Female (%) |
53 (53.0) |
48 (54.5) |
Age, average ± SD |
66.9 ± 14.0 |
66.4 ± 13.9 |
Height, average ± SD |
171 ± 10.4 |
170.7 ± 10.3 |
Weight, average ± SD |
84.5 ± 21.6 |
84.4 ± 21.7 |
BMI, average ± SD |
29.5 ± 7.02 |
29.7 ± 6.85 |
Medication use |
|
|
Less than five medications |
14 (14.0) |
11 (12.5) |
Polypharmacy therapy (☐5 medications) |
86 (86.0) |
77 (87.5) |
OSDI* questionnaire, symptoms of dry eye (score) |
|||||
|
Normal 0–12 points |
Mild 13–22 points |
Moderate 23–32 points |
Severe 33–100 points |
All |
Schirmer-I test |
|
|
|
|
|
Normal Schirmer-I test |
13 (25.5) |
11 (55.0) |
10 (55.6) |
3 (27.3) |
37 (37.0) |
Abnormal Schirmer-I test |
30 (58.9) |
8 (40.0) |
6 (33.3) |
7 (63.6) |
51 (51.0) |
Incomplete Schirmer-I test |
8 (15.6) |
1 (5.0) |
2 (11.1) |
1 (9.1) |
12 (12.0) |
All n (%) |
51 (51.0) |
20 (20.0) |
18 (18.0) |
11 (11.0) |
100 (100) |
Table 2: Number and proportion of patients measured with Schirmer-I test compared to the degree of ocular surface symptoms according to classification of the OSDI questionnaire
|
Less than 5 medicines (<5 medicines) |
Polypharmacy therapy (≥5 medicines) |
All n = 100 |
OSDI symptoms n (%) |
|
|
|
Normal (0–12 points) |
10 (71.5) |
41 (47.7) |
51 (51.0) |
Mild (13–22 points) |
3 (21.4) |
17 (19.8) |
20 (20.0) |
Moderate (23–32 points) |
1 (7.1) |
17 (19.8) |
18 (18.0) |
Severe (33–100 points) |
0 (0.0) |
11 (12.7) |
11 (11.0) |
Schirmer-I test n (%) |
n = 11 |
n = 77 |
n = 88 |
Normal Schirmer-I test |
7 (63.6) |
30 (39.9) |
37 (42.0) |
Abnormal Schirmer-I test |
4 (36.4) |
47 (61.1) |
51 (58.0) |
Excluded |
n = 3 |
n = 9 |
n = 12 |
Incomplete Schirmer-I test |
3 (25%) |
9 (75%) |
12 (100%) |
Table 3: Number and proportion of patients with symptoms of ocular surface symptoms according to the OSDI* questionnaire and normal and abnormal Schirmer-I test, categorized by medicine use
A00-B99 |
Certain infectious and parasitic diseases |
5 |
C00-D49 |
Neoplasms |
1 |
D50-D89 |
Disease of the blood and blood-forming organs and certain disorders |
2 |
E00-E89 |
Endocrine, nutritional and metabolic diseases |
7 |
F01-F99 |
Mental, Behavioral and Neurodevelopmental disorders |
1 |
G00-G99 |
Diseases of the nervous system |
1 |
H00-H59 |
Diseases of the eye and adnexa |
1 |
H60-H95 |
Diseases of the ear and mastoid process |
0 |
I00-I99 |
Diseases of the circulatory system |
6 |
J00-J99 |
Diseases of the respiratory system |
13 |
K00-K95 |
Diseases of the digestive system |
8 |
L00-L99 |
Diseases of the skin and subcutaneous tissue |
6 |
M00-M99 |
Diseases of the musculoskeletal system and connective tissue |
2 |
N00-N99 |
Diseases of the genitourinary system |
10 |
O00-O9A |
Pregnancy, childbirth and the puerperium |
0 |
P00-P96 |
Certain conditions originating in the perinatal period |
0 |
Q00-Q99 |
Congenital malformations, deformations and chromosomal abnormalities |
0 |
R00-R99 |
Symptoms, signs and abnormal clinical and laboratory findings, not |
24 |
S00-T88 |
Injury, poising and certain other consequences of external causes |
11 |
U00-U85 |
Codes for special purposes |
0 |
V00-Y99 |
External causes of morbidity |
0 |
Z00-Z99 |
Factors influencing health status and contact with health services |
2 |






































