Top Links
Journal of Diabetic Complications & Therapy
Comparative Effect of a High Fat with or without High Levels of Sucrose Diets on Peripheral Neuropathy in C57BL/6J Mice
Copyright: © 2021 Yorek MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
objective:Feeding mice a diet containing high fat and high sucrose has been promoted as a good model for type 2 diabetes. This study sought to determine the effect of feeding mice a high fat and high sucrose diet on neuropathy compared to mice fed only a high fat diet and mice fed a high diet and treated with streptozotocin.
Methods:C57Bl/6J mice were divided into five groups and fed the following diets for 20 weeks: Normal (Control); Sucrose enriched (Control + Sucrose), High Fat (Diet-induced obesity (DIO)), High Fat and High Sucrose (DIO + sucrose) and High Fat diet/streptozotocin treated (Diabetic). The endpoints evaluated included motor and sensory nerve conduction velocity, thermal and mechanical sensitivity and innervation of sensory nerves in the cornea and skin.
Results:Diabetic mice were hyperglycemic at the end of the study and along with DIO mice with or without Sucrose had impaired glucose utilization. DIO mice had slowed sensory nerve conduction velocity, mechanical allodynia and decreased innervation of the cornea and skin. DIO + Sucrose and to a greater extent diabetic mice were thermal hypoalgesic, had mechanical allodynia, reduced motor and sensory nerve conduction velocities and decrease innervation of the cornea and skin.
Conclusions:Development of peripheral neuropathy was more severe in High Fat and High Sucrose fed mice compared to high fat fed mice but fasting hyperglycemia and impaired glucose utilization was similar for these two models. Peripheral neuropathy was most severe in diabetic mice.
Keywords: Diet Induced Obesity; Type 2 Diabetes; Peripheral Neuropathy; Western Diet; High Fat; High Sucrose Diet
List of abbreviations:MNCV: Motor Nerve Conduction Velocity; Sec: Second; S.E.M: Standard Error Of The Mean; SNCV: Sensory Nerve Conduction Velocity
There is an ongoing search for rodent models that will closely replicate human diseases in order to determine their etiology and more importantly to identify an effective treatment. This search also applies to obesity and diabetes. My laboratory has focused on the etiology and finding an effective treatment for obesity- and diabetes-induced vascular and neural complications and the lack of a suitable translational model for these conditions has been partially responsible for the lack of discovery of a disease modifying therapy for peripheral neuropathy. There have been several review articles focusing on murine models for the study of diabetic peripheral neuropathy [1,2]. These articles have done a good job presenting the strengths and weaknesses of the different models that are available but few studies have been done comparing outcome measures for peripheral neuropathy in mice models for type 2 diabetes. Numerous studies have been published claiming that high fat fed C57Bl/6J mice are a model for type 2 diabetes [3,4]. However, degree of hyperglycemia in this model is poor with only a very modest increase in fasting blood glucose [5-7]. This led my laboratory and others to perform studies of peripheral neuropathy in high fat fed mice treated with a low dose of streptozotocin that provides a more robust state of hyperglycemia [7-12]. The application of streptozotocin in this manner does not destroy all of the β cells, thus we relate this model to a late stage type 2 diabetes when insulin production has been exhausted and can no longer compensate for the insulin resistant state. However, the use of streptozotocin is troublesome to some investigators when examining the effect diabetes on peripheral neuropathy even though we have previously shown that streptozotocin has no direct impact on peripheral nerve activity [13]. This led us to investigate whether exposing mice to a high fat diet combined with high sucrose would make a more robust model of type 2 diabetes and for peripheral neuropathy compared to high fat diet alone.
Mice fed high fat and high sucrose diets, sometimes referred to as “western” diet have been reported to have a wide array of metabolic related deficits. These include obesity, glucose intolerance and insulin resistance, non-alcoholic fatty liver disease, cardiovascular dysfunction, and cognitive decline [14-21]. However, there is no information available of the impact of a high fat and high sucrose diet compared to a high fat diet alone on the peripheral nervous system. We and others have demonstrated that feeding C57Bl/6J mice a high fat diet causes peripheral neuropathy consistent with a pre-diabetic state. In the present study we examined the effect of a high fat diet combined with high sucrose on peripheral nerve activity and morphometry of small sensory nerves in the skin and cornea.
Unless stated otherwise all chemicals used in these studies were obtained from Sigma-Aldrich Co. (St. Louis, MO).
C57Bl/6J male mice 12 weeks of age were purchased from Jackson Laboratories. Mice were housed in a certified animal care facility and standard diet and water were provided ad libitum. Measures were taken to minimize pain or discomfort and all experiments were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were compliant with all institutional guidelines for use of animals (IACUC approval 1891201). After 1 week on a standard diet (3.0 kcal/g, protein 25.2%, fat 4.4% carbohydrate 39.5% with 13% kcal derived from fat, 7001, Harlan Teklad, Madison, WI) mice were divided into five groups. One group remained on the standard diet and was designated as the Control group. A second group was placed on a Sucrose enriched diet (3.9 kcal/g, protein 19.2%, fat 4.3% carbohydrate 67.3% with 10% kcal derived from fat, D12450J, Research Diets, New Brunswick, NJ). This group was designated as the Control/Sucrose group. The third group was placed on a High Fat diet (5.2 kcal/g, protein 26.2%, fat 34.9%, carbohydrate 25.6%, with 60% kcal derived from fat, D12492, Research Diets). This group was designated as the High Fat group. The fourth group was placed on a High Fat and High Sucrose diet (4.7 kcal/g, protein 20.0%, fat 21%, carbohydrate 50%, with 40% kcal derived from fat, D12079B, Research Diets). This group was designated as the High Fat and High Sucrose group. The fifth group was placed on a High Fat diet like group 3 and after 8 weeks on this diet were treated with 100 mg/kg streptozotocin (EMD Chemicals, San Diego, CA) followed three days later if needed with a second dose of streptozotocin (50 mg/kg) as previously described [5]. This group was designated as the Diabetic group. All diabetic mice had a blood glucose ≥13.8 mM (250 mg/dl) (Accu-Chek, Roche Inc., Indianapolis, IN). These dietary/disease conditions were continued for a period of 20 weeks. Diet consumption was examined between weeks 3-5 and 7-9. Diet consumption for Control, Control + Sucrose, High Fat, High Fat + High Sucrose and Diabetic mice were 68.5 ± 5.2, 60.3 ± 6.1, 58.3 ± 4.8, 59.4 ± 4.4 and 63.1 ± 5.1 g/kg/day, respectively
Glucose utilization was determined by injecting mice with a saline solution containing 2 g/kg glucose, i.p., after an overnight fast as previously described [11]. Circulating blood glucose levels were measured immediately prior to the glucose injection and at 15, 30, 45, 60, 90 and 120 min afterwards.
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method with instrumentation provided by IITC Life Science; Woodland Hills, CA (model 390G). The mouse was placed in the observation chamber on top of the thermal testing apparatus and allowed to acclimate to the warmed glass surface (30oC) and surroundings for a period of 15 min. The mobile heat source was maneuvered so that it was under the heel of the hindpaw and then activated, a process that activates a timer and locally warms the glass surface, when the mouse withdrew its paw, the timer, and the heat source was turned off [5]. Following an initial recording, which was discarded, four measurements were made for each hindpaw, with a rest period of 5 min between each set of measurements. The mean of the measurements, reported in seconds, was used as a measure of the thermal nociceptive response latency. Tactile responses were evaluated by quantifying the withdrawal threshold of the hindpaw in response to stimulation with flexible von Frey filaments as previously described [22]. The data were reported in grams. Each of these tests was repeated at least three times with a rest period of 10 minutes between tests. These tests were performed in a masked manner and completed immediately before the terminal procedures on different days.
Mice were anesthetized with Nembutal (75 mg/kg, i.p., Abbott Laboratories, North Chicago, IL) and motor and sensory nerve conduction velocities were determined as previously described [5,11]. Core temperature was monitored using a rectal probe and temperature regulated between 36oC and 37oC using a heating pad and radiant heat. The data were reported in m/sec.
Sub-epithelial corneal nerves were imaged using the Rostock cornea module of the Heidelberg Retina Tomograph confocal microscope as previously described [22,23]. Prior to experimentation, the anesthetized mouse was secured to a customized platform that allowed adjustment and positioning in three dimensions. Five random high-quality images, without overlap of the sub-epithelial nerve plexus of the central cornea, were acquired by finely focusing the objective lens to maximally resolve the nerve layer just under the corneal epithelium. The investigator acquiring these images was masked with respect to identity of the mice condition. The corneal nerve fiber length was reported as the total length of all nerve fibers and branches (in millimeters) present in the acquired images standardized for the area of the image (in square millimeters). The corneal fiber length for each animal was the mean value obtained from the acquired images and expressed as mm/mm2. Based on receiver operating characteristic curve analysis, corneal nerve fiber length is the optimal parameter for diagnosing patients with diabetic neuropathy and has the lowest coefficient of variation [24]
As previously described, immunoreactive nerve fiber profiles innervating the skin of the hindpaw were determined using standard confocal microscopy [21,25]. Samples were surgically acquired, fixed and the immunostained nerve profiles counted by two individual investigators that were masked to the sample identity. All immunoreactive profiles were normalized to the length epidermal specimen.
Non-fasting blood glucose was determined using a glucometer (Accu-Chek, Roche Inc., Indianapolis, IN). Serum samples were collected for determination of triglyceride and free cholesterol using commercial kits from Sigma-Aldrich Co., St. Louis, MO and BioVision, Mountain View, CA, respectively. To examine steatosis, liver samples were frozen in OCT compound (Sakura FineTek USA, Torrance, CA) in liquid nitrogen. Liver sections, 5 µm, were incubated with BODIPY (Molecular Probes, Carlsbad, CA), at a 1:5,000 dilution in 1% bovine serum albumin for 1h at room temperature. After washing liver sections were mounted using ProLong® Gold antifade reagent (Molecular Probes, Carlsbad, CA) and covered with a glass coverslip. Images were collected using Zeiss 710 LSM confocal laser scanning microscope. Images were analyzed for % area fraction of lipid droplets using Image J software [25].
Results are presented as mean ± S.E.M. Comparison between control, non-treated and treated diabetic mice were conducted using one-way ANOVA and Bonferroni post-hoc test comparison (Prism software; GraphPad, San Diego, CA). A P value of less than 0.05 was considered significant.
Data in Table 1 provides the beginning and end weight for the mice in the five study groups. Data in this table also reveals that only the Diabetic mice were hyperglycemic at the end of the study period. Serum cholesterol was significantly increased in the High Fat fed, High Fat and High Sucrose fed and Diabetic mice compared to Control mice. The cholesterol levels in the High Fat + High Sucrose fed mice was also significantly higher compared to High Fat fed and Diabetic mice. Serum cholesterol levels were also higher in Control mice fed a diet containing elevated Sucrose compared to Control mice but this difference did not reach significance. Serum triglyceride levels trended to be increased in mice fed the High Fat + High Sucrose diet as well as in Diabetic mice but the difference did not reach significance compared to mice fed the Control diet. Non-alcoholic fatty liver analysis was performed and data presented in Table 1. Mice fed the Control and Control + Sucrose diets had a low abundance of fat in the liver. In contrast, the fatty liver (steatosis) was significantly increased in mice fed the High Fat and High Fat + Sucrose diets and with Diabetes.
Even though only Diabetic mice had a non-fasting hyperglycemic state data in Figure 1 demonstrate that High Fat fed and High Fat + High Sucrose fed mice as well as Diabetic mice had a significantly elevated fasting blood glucose (Control: 103 ± 8; Control + Sucrose: 94 ± 6; High Fat: 185 ± 12; High Fat + High Sucrose: 175 ± 9; Diabetic: 301 ± 24) and impaired glucose clearance compared to mice fed a Control or Control + Sucrose diet.
Figure 2 provides data for motor and sensory nerve conduction of mice in the five treatment groups. Motor and sensory nerve conduction velocities in mice fed the Control + Sucrose diet were similar to mice receiving only Control diet suggesting that an increase in dietary carbohydrate does not impact nerve conduction velocity in otherwise healthy mice. As previously demonstrated mice fed a High Fat diet for 20 weeks have a normal motor nerve conduction velocity but an impaired sensory nerve conduction velocity compared to Control mice [5,7]. When a high level of Sucrose was added to the High Fat diet the mice receiving this diet had a significantly impaired motor and sensory nerve conduction velocity compared to control mice. However, the difference in motor nerve conduction velocity between mice fed the High Fat diet and the High Fat + High Sucrose diet were minimal with the latter being significantly different compared to control mice. Motor nerve conduction velocity was not significantly impaired when compared to mice fed the Control diet enriched with Sucrose. Sensory nerve conduction velocity in mice fed the High Fat + High Sucrose diet was significantly impaired vs. mice fed the Control diet and mice fed the Control + Sucrose diet. As previously reported motor and sensory nerve conduction velocities were significantly decreased in mice representing a late stage 2 of diabetes compared to Control mice [5,7,8].
Mice fed a Control diet or this diet supplemented with Sucrose had very similar sensory nerve density profiles in cornea and intraepidermal nerve fibers (Figure 3). Feeding mice a High Fat diet or a High Fat + High Sucrose diet resulted in a significant decrease that was similar between the two sets of mice in cornea nerve fiber length and intraepidermal nerve fiber density compared to mice fed the Control and Control + Sucrose diets. In Diabetic mice sensory nerve fiber density in the cornea and skin was also significantly decreased compared to Control mice with the decrease in intraepidermal nerve fiber density being significantly greater in Diabetic mice compared to mice fed a High Fat diet or High Fat + Sucrose diet.
We examined behavioral responsiveness of the skin to both a thermal and mechanical stimulus (Figure 4). Thermal nociception and mechanical allodynia were similar for mice fed the Control diet or the Control + Sucrose diet. Latency for thermal sensitivity was increased similarly in mice fed the High Fat and the High Fat + High Sucrose diets but this obtained significance to Control for only the High Fat + High Sucrose fed mice when compared to mice fed the Control diet or the Control + Sucrose diet. Mechanical allodynia was also similar for both groups and was significant compared to mice fed the Control diet or the Control + Sucrose diet as well as to Diabetic mice. The change in latency to a thermal stimulus and mechanical allodynia was greatest in Diabetic mice.
The primary finding was other than a small difference in the impairment of motor nerve conduction velocity and intraepidermal nerve fiber density in High Fat + High Sucrose fed mice compared to High Fat fed mice the peripheral neuropathy created by 20 weeks of these diets were similar. These results also confirmed our previous studies that a more severe hyperglycemic state as observed in our Diabetic group results in a more extensive peripheral nerve damage that includes significant slowing of motor nerve conduction velocity and impairment in sensitivity and density of sensory nerve fibers in the skin and cornea. Additional interesting outcomes was finding that increasing the carbohydrate/sucrose content of the control diet (Control + Sucrose) did not impact glucose utilization or peripheral neuropathy endpoints compared to mice fed a Control diet alone. Other interesting results were the significantly higher serum cholesterol level that occurred with mice fed the High Fat + High Sucrose diet compared to mice fed the High Fat diet alone or Diabetic mice, whereas liver steatosis was similar for High Fat, High Fat + Sucrose and Diabetic mice. Increased levels of cholesterol could have an impact on vascular reactivity. However, this possibility was not addressed in this study and no analyses relating to vascular function were performed. The last interesting result, although not surprising, was that glucose utilization and peripheral neuropathy related endpoints were most impacted by diabetes created by treating high fat fed mice with a low dose streptozotocin. In this study we did not treat mice fed a High Fat + High Sucrose diet with a low dose streptozotocin. The purpose of the study as discussed above was to compare peripheral neuropathy outcome measures between High Fat fed mice and mice fed a High Fat + High Sucrose diet. Our hypothesis was that glucose disposal, insulin resistance and peripheral neuropathy would be more severe in the mice fed the High Fat + High Sucrose diet compared to High Fat diet alone but this was not the case. Creating a diet having a High Fat content along with high carbohydrate content does not appear to create a poorer metabolic condition that would cause a significantly more severe state of peripheral neuropathy than what occurs with a high fat diet alone.
In other related studies Baranowski et al. [26] showed that feeding C57Bl6/J mice a High Fat and High Sucrose diet caused increased inflammation and energetic stress in the hippocampus and prefrontal cortex as well as cognitive decline. Feeding C57Bl6/J mice High Fat and High Sucrose diet has also been shown to cause activation of macroglia and damage to retinal ganglion cells [27]. Both of these studies claimed the effects of the High Fat and High Sucrose diet was independent of hyperglycemia. However, neither study examined the effect of a High Fat diet alone. Moreover, our studies are among the first to examine as well as compare the effects of a High Fat and High Sucrose diet on peripheral neuropathy to a High Fat diet alone.
Lang et al [28] fed C57Bl/6J mice for 12 weeks a standard chow diet, cafeteria diet or high fat diet. They reported that the cafeteria and high fat diets induced visceral obesity, glucose intolerance and insulin resistance. Fasting blood glucose was minimally increased in mice fed the cafeteria and high fat diet similar to our finding. They also found that the cafeteria diet was more effective than the high fat diet in causing aorta vascular dysfunction while hypercoagulability was mostly evident in mice fed the high fat diet. In a study using 8 week old Sprague-Dawley rats fed a cafeteria diet for 13 weeks it was found that these rats demonstrated prediabetes that included a minimally elevated fasting blood glucose, obesity, impaired glucose tolerance and dyslipidemia [29]. They found motor and sensory nerve conduction velocities, tactile sensitivity and intraepidermal nerve fiber density to be unchanged but nerve superexcitability was significantly increased in cafeteria fed rats compared to control rats. However, these investigators did not perform any studies with high fat fed rats. Their data also differs from our reported results with high fat fed SpragueDawley rats. We have shown that feeding 12 week old Sprague-Dawley rats a high fat diet for 12 weeks causes obesity, impairment of glucose clearance, slowing of sensory nerve conduction velocity but not motor nerve conduction velocity and decrease in sensitivity and density of cornea and intraepidermal nerve fibers [30-33]. Our results in high fat fed rats are similar to the results reported in this study in C57Bl6/J mice fed a high fat diet. Overall, these and other studies imply that different types of diets can induce obesity that is often accompanied by insulin resistance and steatosis, however; the development and progression of other complications including peripheral neuropathy vary. This may be due to a variety of causes including age of the animal, duration of treatment and the impact of the metabolic derangement on function of peripheral tissues including nerves.
Limitations of the study as mentioned above are that we did not include a study group of High Fat + High Sucrose fed mice treated with streptozotocin. It is possible that this combination could create a more severe diabetic state due to the increased carbohydrate in the diet. Greater significance between the High Fat and High Fat + High Sucrose fed groups may have been realized if a larger cohort were studied. However, power analysis confirmed that an n of 12 per group would be sufficient based on our primary outcome measures. An n of 12 per group was also consistent with our previous studies. Strengths of the study include diets were obtained from commercial sources; analyses were performed in a masked approach and the experience of the study team.
High Fat fed mice as well as High Fat + High Sucrose fed mice are both considered to be rodent models for pre-diabetes. Common characteristics of these two models are obesity, insulin resistance and liver steatosis. Fasting blood glucose levels are significantly increased above control values in both of these models but the increase is minimal and it is unlikely that these models provide a good representation of type 2 diabetes. Moreover, it is unknown whether one of these models may be better than the other. In this study we demonstrated that impaired glucose clearance and several endpoints relating to peripheral neuropathy were similarly impacted by feeding mice either a High Fat diet or a High Fat + High Sucrose diet. We conclude that for studies relating to the effect of pre-diabetes on peripheral neuropathy either model is appropriate.
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development (RX000889-01), Iowa City VA Center of Excellence for the Prevention and Treatment of Visual Loss: (C9251-C) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107339 from NIH. The content of this manuscript are new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies.
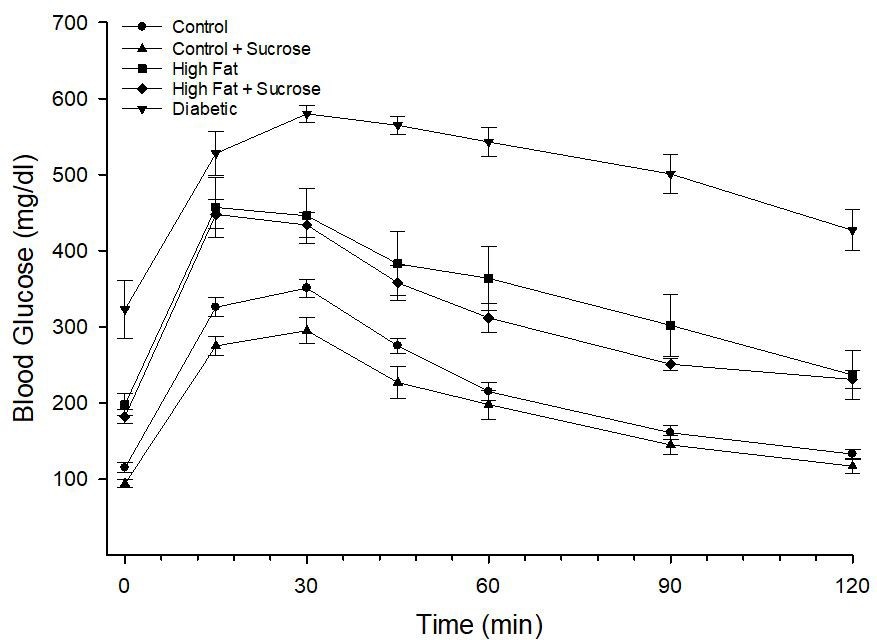 |
| Figure 1: Glucose utilization curve for mice fed Control, Control + Sucrose, High Fat, High Fat + High Sucrose diets and Diabetic mice. Data are the mean ± S.E.M. The area under the curve (AUC) was significantly different, p < 0.05 (impaired), mice fed the High Fat and High Fat + High Sucrose diets and p < 0.01 (impaired) for Diabetic mice vs. fed a Control or Control + Sucrose diet. The number of mice in each group was the same as shown in Table 1 |
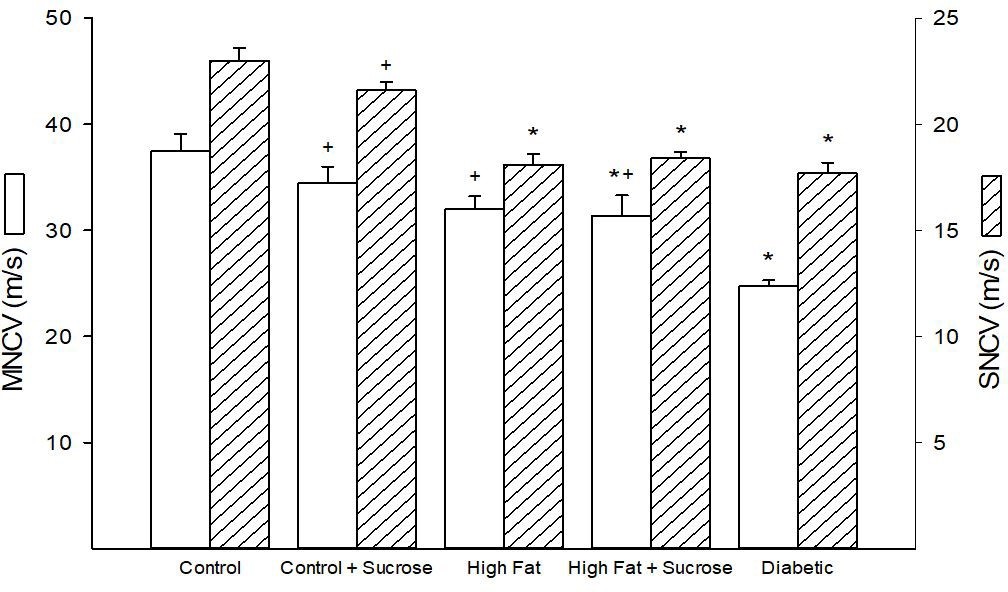 |
| Figure 2: Motor and sensory nerve conduction velocity for mice fed Control, Control + Sucrose,
High Fat, High Fat + High Sucrose diets and Diabetic mice. Motor and sensory nerve conduction
velocities were determined as described in the Materials and Methods section. Data are presented
as the mean ± S.E.M. in m/sec. The number of mice in each group was the same as shown in Table 1. * p < 0.05 compared to mice fed the Control diet; + p < 0.05 compared to Diabetic mice |
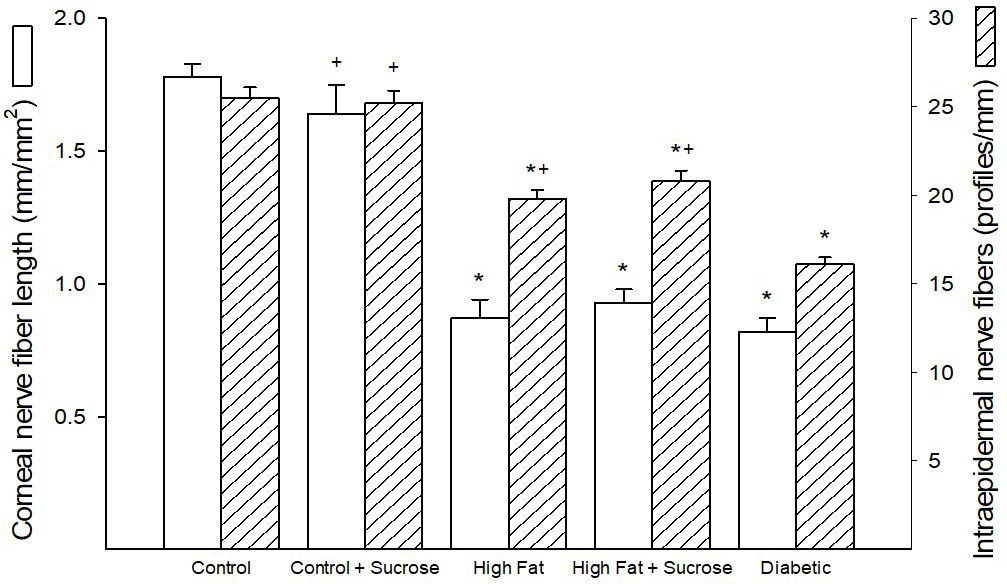 |
| Figure 3: Corneal nerve fiber length and intraepidermal nerve fiber density for mice fed Control, Control + Sucrose, High Fat, High Fat + High Sucrose diets and Diabetic mice. Corneal nerve fiber length and intraepidermal nerve fiber density were determined as described in Materials and Methods section. Data are presented as the mean ± S.E.M. in mm/mm2 and profiles/mm, respectively. The number of mice in each group was the same as shown in Table 1. * p < 0.05 compared to mice fed the Control diet; + p < 0.05 compared to Diabetic mice |
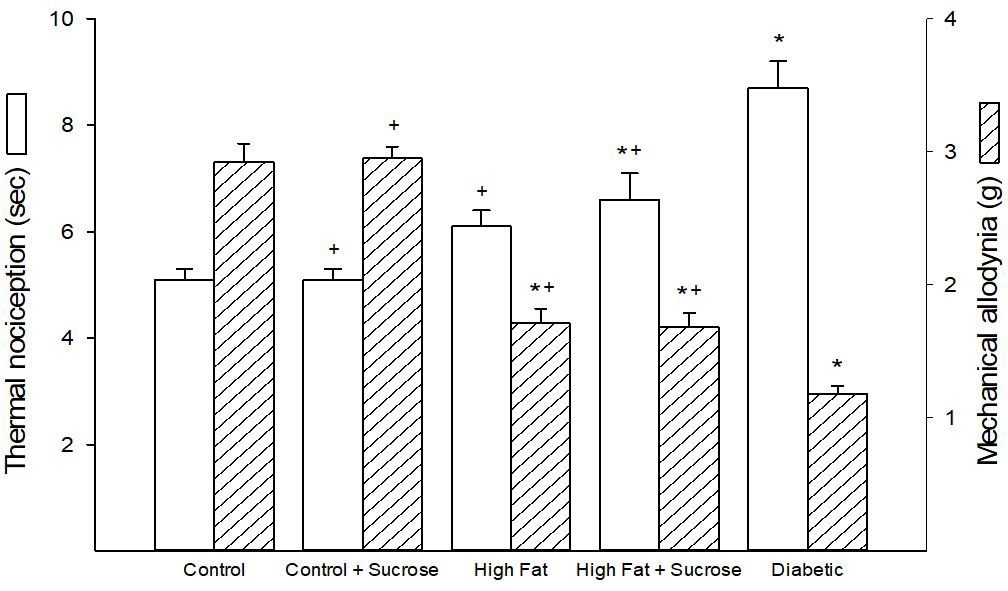 |
| Figure 4: Thermal nociception and mechanical allodynia for mice fed Control, Control + Sucrose, High Fat, High Fat + High Sucrose diets and Diabetic mice. Thermal nociception and mechanical allodynia were determined as described in Materials and Methods section. Data are presented as the mean ± S.E.M. in seconds (sec) and grams (g), respectively. The number of mice in each group was the same as shown in Table 1. * p < 0.05 compared to mice fed the Control diet; + p < 0.05 compared to Diabetic mice |
Condition |
Start wt (g) |
End wt (g) |
Blood glucose (mg/dl) |
Serum Cholesterol (mg/ml) |
Triglyceride (mg/ml) |
Steatosis (%) |
Control (12) |
26.5 ± 0.4 |
30.7 ± 0.4 |
206 ± 14 |
1.5 ± 0.2 |
0.61 ± 0.03 |
5.7 ± 0.5 |
Control + Sucrose (10) |
26.6 ± 0.4 |
33.2 ± 0.8 |
239 ± 15b |
2.6 ± 0.3 |
0.55 ± 0.05 |
5.7 ± 0.4 |
High Fat (11) |
27.6 ± 0.7 |
48.5 ± 1.7a,b |
229 ± 11b |
3.7 ± 0.3a |
0.64 ± 0.07 |
39.1 ± 1.8a |
High Fat + High Sucrose (12) |
27.8 ± 0.4 |
44.6 ± 2.2a,b |
219 ± 19b |
5.4 ± 0.6a,b,c |
0.77 ± 0.11 |
37.3 ± 1.6a |
Diabetic (12) |
26.4 ± 0.4 |
35.9 ± 2.5 |
468 ± 22a |
3.3 ± 0.3a |
0.77 ± 0.10 |
45.3 ± 1.7a |
c: P < 0.05 compared to High Fat. Parentheses indicate the number of experimental animals.
Table 1: Effect of High Fat Diet, High Fat/High Sucrose Diet or Type 2 Diabetes on Weight, Blood Glucose, and Serum Cholesterol and Triglyceride Levels and Liver Steatosis in C57Bl/6J Mice






































