Top Links
Journal of Advancements in Food Technology
ISSN: 2639-3328
The Sensory Attributes Potentiating Effect of Mid-Infrared Rays on Moringa Leaf
Copyright: © 2024 Umakanthan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Moringa leaf (Moringa oleifera) is referred to as the “natural nutrition of the tropics”, and in the Philippines, as “mothers’ best friend”. It possesses many health benefits and is also used in disease prevention and therapy. The enhancement of moringa’s natural potency is a scientific challenge that has yet to be thought of. We found enhanced inherent characteristics in the moringa leaf by irradiating using our recently invented 2-6µm mid-infrared ray generating atomizer (MIRGA). The achieved benefits were a more desirable taste and aroma, and an enhanced shelf life, thus increasing the potential transportation distance, quality, and palatability. This scientific achievement was supported by suitable instrumentations and sensory evaluation.
Keywords: MIRGA; 2-6 µm mid-IR; Moringa leaf; Inherent Characters; Potentiation; Benefits
Moringa oleifera is a tropical tree. Moringa leaves are useful for multiple therapies and other medical and food applications [1]. They are rich in bioactive compounds such as phenolic, flavonoids, protein, polysaccharides, vitamins, and minerals, which exhibit various activities including anti-inflammatory, anti-tumour, antibacterial, antioxidant, and anti-diabetic properties. Additionally, Moringa leaves are a good source of essential nutrients, growth factors, vitamins, amino acids, proteins, and minerals, making them a potential ingredient for nutritional supplements and medicine. The demand for Moringa leaves is increasing, and they are being used in the food industry as a source of nutrients and antioxidants.
And, consumer acceptability of food depends on taste, convenience, health-enhancing properties, and shelf life. Sensory characteristics of foods, such as liking and palatability, play a crucial role. The acceptability limit is an important parameter in determining shelf life, as it considers the potential negative consequences of food unacceptability in the market. Therefore, both taste and shelf life are key factors in consumer acceptability of food.
But, moringa leaves have a short shelf life [2], hence the ability to endure long-distance transportation is limited. Although the leaves have multiple health benefits, their slightly bitter taste partially restricts their use for human consumption, and thereby limits their applications in nutraceutical products. Hence, increasing their shelf life and palatability is essential, so that they can be transported long distances, especially for use in cold-climate countries, and to consume with interest. In this study, we applied the non-ionizing, biologically safe 2-6 µm mid-IR which can penetrate most intervening media [3,4]. Here we have detailed the sensory taste study conducted, shelflife observation, and the effects of mid-IR in the chemistry of moringa leaves before and post mid-IR irradiation.
Mid-infrared Generating Atomizer – MIRGA (patent no.:401387) is a 20 ml capacity polypropylene plastic atomizer containing inorganic (molar mass 118.44 gm/mole) water-based solution (having approximately two sextillion cations and three sextillion anions). Dimensions of the unit are 86 x 55 x 11 mm, orifice diameter 0.375 mm, ejection volume 0.062 + 0.005 ml, ejection time 0.2 sec and average pressure 3900 pascal and cone liquid back pressure 2000 N/m2 (Supplementary figure F1). During spraying, approximately 1 μg weight of water as the mist is lost and the non-volatile material in the sprayed liquid is 153 mg/ml. Every time spraying emits 0.06ml which contains approximately seven quintillion cations and eleven quintillion anions. Depending on the pressure (varies with the user) applied to the plunger, every spraying is designed to generate 2-6 µm mid-infrared which was estimated by FTIR (retro-reflector) interferometer instrument (Detector type D* [cm HZ1/2 - 1] MCT [2-TE cooled]) at Lightwind, Petaluma, California. (Raw data in Supplementary Data D1) [5].
Spraying is done from a distance of 0.25 to 0.50 meters externally over packaged (e.g. polythene, paper) moringa leaves (method of spraying in Supplementary video V1). This distance is essential for the MIRGA-sprayed solution for the formation of ion clouds, oscillation, and 2-6µm mid-IR generation (refer to Discussion). The mid-IR penetrates the intervening package and acts on the inside Moringa leaves. Close spraying never generates energy. Generally, MIRGA should be used as a body spray.
Green leaves plucked from the moringa tree were used. Besides housewives and moringa leaf merchants, a separate trained sensory expert panel (n=6) was also employed to evaluate the sensory attributes of the leaves.
The instruments used to identify the Chemical compound transformation: Chemical compound transformation – Gas chromatography–mass spectrometry (GC-MS); Chemical bond changes – Fourier transform infrared spectroscopy (FTIR); Structural changes – Powder X-ray diffraction (PXRD); Proton resonances – Proton nuclear magnetic resonance (1H-NMR).
Moringa leaves (500 g) were collected from a matured Moringa oleifera tree and packed in a polythene bag sealed with cellophane tape, but left with a small opening to remove the leaves for sensory testing during the trial. To start with, the control (non-sprayed) leaves were tasted by the sensory panel. The expert panel individually recorded their sensory score using a 9-point hedonic scale: 1- Dislike extremely, 2 - Dislike very much, 3 - Dislike moderately, 4 - Dislike slightly, 5 - Neither like nor dislike, 6 - Like slightly, 7- Like moderately, 8 - Like very much, 9 - Like extremely [6,7], whereas the other tasters recorded their opinions as ‘like’ or ‘dislike’. After the initial evaluation, the bag was given one MIRGA spraying on one side, and sensory evaluation was recorded, followed by 2nd spraying to another side of the packet followed by sensory evaluation. In this manner, the spraying was given one by one on the alternative sides of the packet, followed by sensory evaluation each time, and like this 8 sprayings were given. After the 8th spraying, the leaves became unpalatable, i.e. the leaves completely lost their inherent character, and the spraying was stopped. It is noted that after 4th spraying, the leaves showed significantly desirable sensory attributes.
Later, based on the above method, leaves from the same tree were collected, subjected to 0, 4, or 8 MIRGA sprayings, cooked, and tasted. To estimate shelf life, the 0, 4, and 8 times sprayed leaves were kept at room temperature (28–390 C) and some in a refrigerator. The control, 4, and 8 times sprayed leaves were dried, powdered, and used for various instrumentations; these samples were from the same tree, the difference between them being only the number of sprayings they received. Similarly, moringa leaves from 8 trees within a radius of 25 km were tested.
FTIR: A small quantity of the sample is added to KBr in the ratio of 1:100 approximately. The matrix was ground for 3-4 minutes using mortar and pestle. The fine powder is transferred into a 13 mm diameter die and made into a pellet using a hydraulic press by applying a pressure of 7 tonnes. The fine pellet is subjected to FTIR analysis using a universal pellet holder. Infrared spectral data were collected on the ThermoAvtar 370 FTIR spectrometer. Spectra are collected over a range of 4000–400 cm−1 at 4 cm−1 resolution with an interferogram of 32 scans.
PXRD: The sample is smeared over a low background sample holder (amorphous silica holder) and fixed on the sample stage in a goniometer. The instrument is set with B-B geometry. The current and voltage are set to 40 mV and 35 mA and data have been collected. Instrument maker: Bruker Model D8 Advance Goniometer: theta/2 theta.
TEM: An extremely small amount of material is suspended in water/ethanol (just enough to obtain a slightly turbid solution). The solution is homogenized using an ultrasonicator to disperse the particles, a drop of the solution is then pipetted out and cast the drop on carbon-coated grids of 200 mesh the grid is dried and fixed in the specimen holder-instrument Maker: Jeol Model JM 2100.
NMR: The experiments were done on a 600 MHz NMR spectrometer (ECZR Series, JEOL, JAPAN) using a 3.2mm CPMAS probe at 150MHz frequency. All the samples were run at 18KHz spinning speed at room temp and with a delay of 5sec.
Compared to raw and cooked control leaves, the 4 times sprayed leaves have acquired a better taste, aroma, palatability, and shelf life (100–150% at room temperature and 300-500% in refrigeration), whereas 8 times sprayed leaves were unpalatable and had a reduced shelf life than control (Table 1). The sensory attribute changes were perceived in 1-5 minutes after spraying
The housewife and merchants very much liked the 4 times sprayed leaves than the control and extremely disliked the 8 times sprayed leaves (both the raw and cooked).
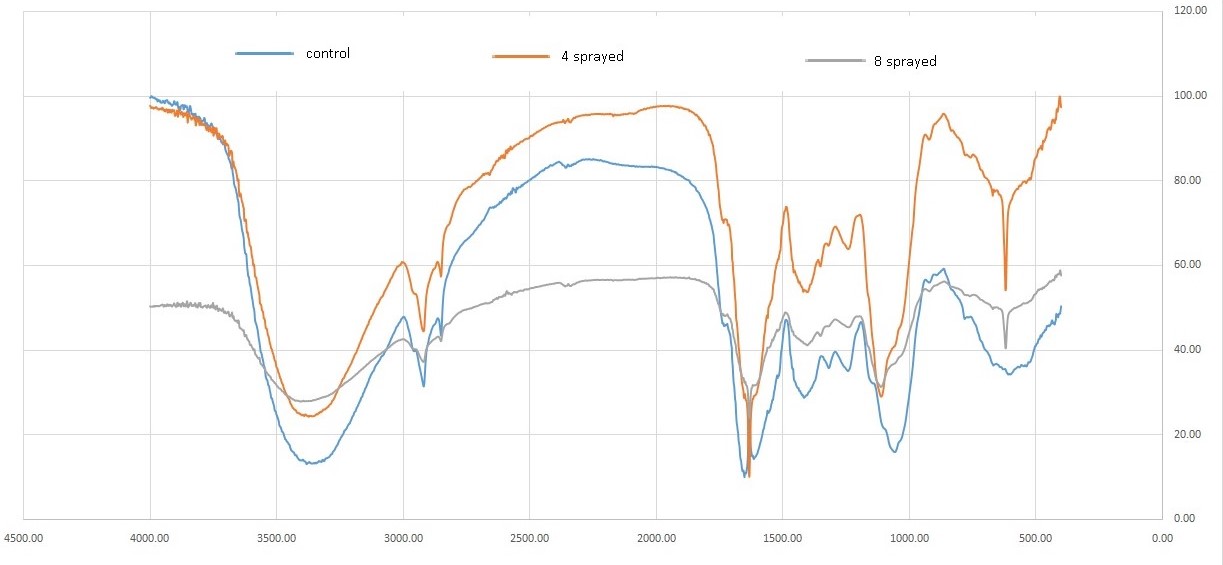
Peak 3367 cm-1 is responsible for the presence of the O-H here we can determine it as part of the water peak and OH group from carbohydrates. Compared to the control, in 4 sprayed samples square of this peak is bigger by 110%. In 8 sprayed sample squares this peak is bigger by 206%. Peak 2926 cm-1 is responsible for the presence of the C-H and is characteristic of the carbon chain. Compared to the control, in 4 sprayed samples square of this peak is bigger by 113%. In 8 sprayed sample squares this peak is bigger by 182%. Peak 2855 cm-1 is responsible for the presence of the C-H and is characteristic of the carbon chain. Compared to the control, in 4 sprayed samples square of this peak is bigger by 100%. In 8 sprayed sample squares this peak is bigger by 120%. Peak 1742 cm-1 is responsible for the presence of the C=0 here we can determine it as part of lipids. Compared to the control, in 4sprayed samples square of this peak is bigger by 106%. In 8 sprayed sample squares this peak is bigger by 234%. Peak 1650 cm-1 is responsible for the presence of the N-H here we can determine it as part of proteins. Compared to the control, in 4 sprayed samples square of this peak is bigger by 109%. In 8 sprayed sample squares this peak is bigger by 252%. Peak 1630 cm-1 is responsible for the presence of the N-H here we can determine it as part of proteins. Compared to the control, in 4 sprayed samples square of this peak is bigger by 148%. In 8 sprayed sample squares this peak is bigger by 27%. Peak 1610 cm-1 is responsible for the presence of the N-H here we can determine it as part of proteins. Compared to the control, in 4 sprayed samples square of this peak is bigger by 110%. In 8 sprayed sample squares this peak is bigger by 197%. Peak 1150 cm-1 is responsible for the presence of the C-O group here we can determine it as part of carbohydrates. Compared to the control, in 4 sprayed samples square of this peak is bigger by 100%. In 8 sprayed sample squares this peak is bigger by 55%. Peak 1050 cm-1 is responsible for the presence of the C-O group here we can determine it as part of carbohydrates. Compared to the control, in 4 sprayed samples square of this peak is bigger by 101%. In 8 sprayed sample squares this peak is bigger by 230%. (Figure 1)
The area of peaks observed in the FTIR spectra increased for the 4 times sprayed sample caused by protein and carbohydrate restructuring. The 8 times sprayed sample showed a much greater increase in the peaks which led to changes beyond the desirable limit [8]. Thus, the 4 times the sprayed sample is more favorable and the 8 times sprayed is less favorable compared to the control.
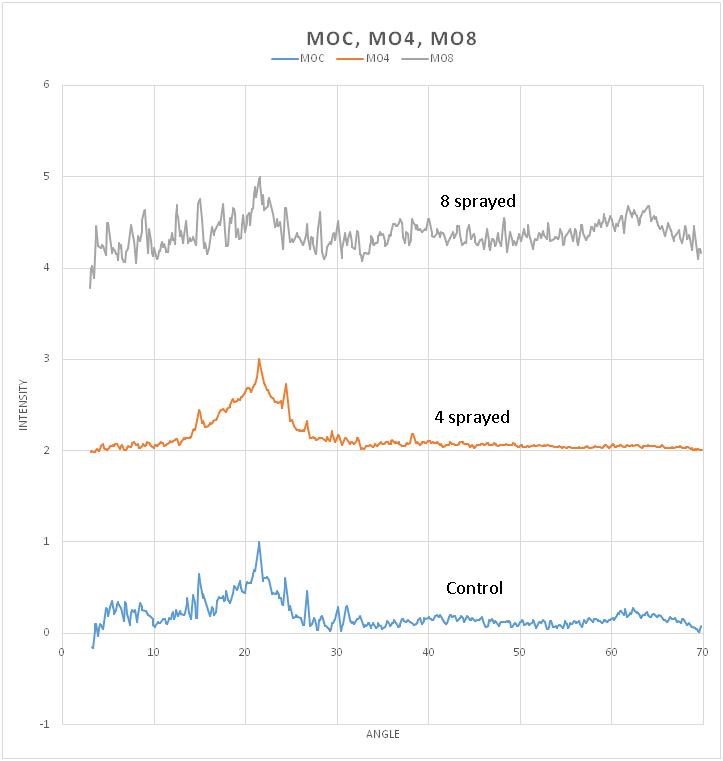
Control sample: A broad, diffused peak between 2 = 16o and 26o, centered on 21.50 o is observed. Several intervening strong peaksare observed at 2 = 14.94 o, 24.28o , and 26.70 o. A doublet or split-peaks can be seen at 2= 30.96o and 31.04o. Other minor peaks are observed from 2 = 5.43o to 8.47o. 4 sprayed sample: A broad, diffused peak between 2= 10o and 30 o, centered on 21.54 o is observed.Several overriding peaks are observed at 2= 14.96 o, 24.47o , and 26.72 o . Other minor peaks are observed from 2 = 29.41o to 32.28o.Another minor peak is observed at 38.26o. 8 sprayed sample: 8 sprayed sample’s diffraction pattern shows a very low signal-- to-noise ratio. One discernible peak at 2 = 21.55 o is seen.
All three samples showed a broad peak at 21.5o shifting to higher 2 values with the increase in spraying. The same is observed for the peaks that are both present in control to 4 times sprayed with the increased spraying number. Control and 4 times sprayed samples have very close structures as shown by the present peaks at 14.9o,21.5o,24.5o, 26.7o, and 30o. These peaks also correspond to the peaks identified in the literature . The control sample showed more minority peaks than the 4 times sprayed sample, namely the peaks at 5o, 8o,31o, 62o, and 63o. The breadth of peak centered at 21.5o decreases from XRD data of control to 4 times sprayed. Crystalline peaks can also be easily discerned in 4 times sprayed than control. 4 times sprayed shows a good signal-to-noise ratio and more defined peaks but most of its reflection peaks overlap with the broad peak. 8 times sprayed samples showed very poor signal-- to-noise ratio and poorly resolved peaks. This indicates that the predominant phase is amorphous. (Figure 2)
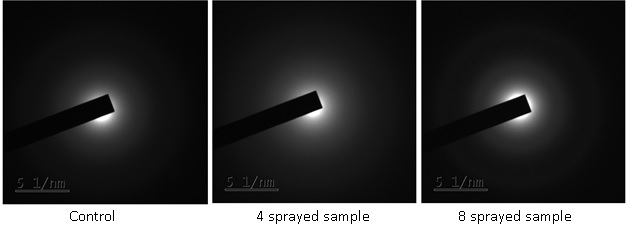
Patterns of the three samples show concentric diffuse areas with no bright spots or discrete rings, indicating that the control and sprayed samples have an amorphous texture. MIRGA sprayings have not significantly affected the atomic arrangement of the moringa leaf powder matrix (Figure 3).
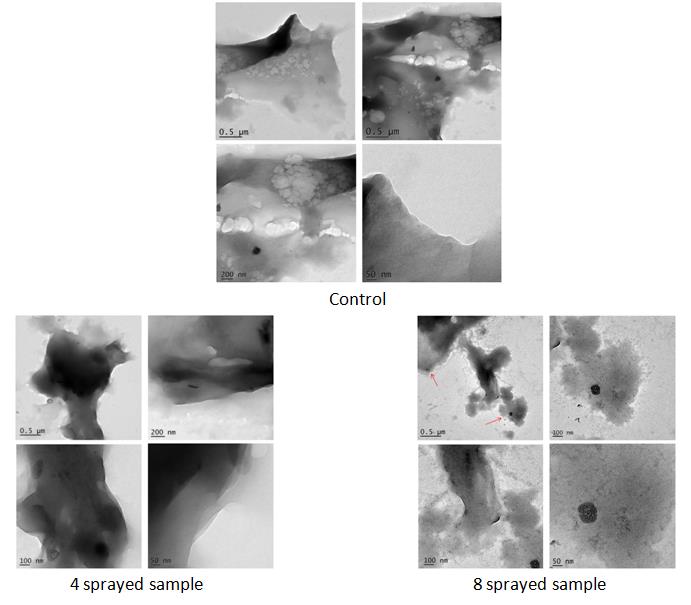
Control sample: has amorphous-shaped fragments in the micrometer scale and far above 1 µm. The surface of fragments appears smooth and the material composing it is distributed homogeneously along the fragment structure, with the only exception of parts where holes are located. Dark particles visible in the images range in the 20 – 50 nm interval and appear either semi-spherical or ellipsoidal. Details of the fragment edge confirm the compact and homogeneous distribution of the material composing the fragment itself.
4 sprayed samples have amorphous-shaped fragments with sizes in the micrometer scale and far above 1 µm. The surface of fragments appears smooth, but differently from the control, the material composing it is distributed unevenly along the fragment structure. In the fragment structure, indeed, some areas appear darker than others. 4 spraying has caused a mass rearrangement within the fragment body. This also explains the absence of areas with holes, observed instead in the control. The fragment edge itself appears layered, differently from the compact aspect of the control.
8 sprayed samples have amorphous-shaped fragments ranging from 1.6 x 0.3 µm2, while smaller ones range from 0.5 – 1 µm on average. The surface of fragments appears misty, diffuse, and cloudy, largely different from both the control and 4 sprayed samples. Similar to 4 sprayed samples, but more intensively, the material composing fragments are distributed unevenly along the body structure. In the fragment structure, indeed, some areas appear darker than others. Dark particles are visible, smaller ones range from 20 – 40 nm, while larger ones are around 100 nm.
It is inferred from the TEM analyses that mid-infrared generated from MIRGA spraying acted on the chemical bonds and has effected changes in the comprising fragments of moringa leaves (Figure 4).
δ 7.3 (s, 1H), δ 7.0 (s, 1H), 6.1 (s, 1H), 5.4 (m, 1H), 5.2 (d, 1H), 4.9 (t, 1H), 4.5 (m, 1H), 4.2 (t, 1H), 3.5 (H2O), 2.5 (DMSO), 2.2 (m, 3H), 2.0 (m, 1H), 1.6 (m, 1H), 1.2 (d , 2H), 0.9 (d, 3H), 0.7 (d, 3H)
The three samples have more or less similar peak assignments. The 1H NMR spectra reveal the presence of a three-proton singlet at δ 2.2 for a CH3 group on an aromatic ring, two peaks each of three-proton intensity at δ 0.7 and 0.9 for CH3. It also shows the CH2 group at δ 1.2 and two isolated aromatic singlets at δ 6.9 and 7.6 (each 1H). The CH3 group resonances are attributed to the different CH3 groups. The peak at δ 6.1 corresponds to the CH3–CO-. The sharp peak at δ 2.5 is due to the contamination in the solvent (DMSO) and the broad peak at about 3.5 is due to water. (Table 2) (Fig 5)
To distinguish between the 3 subsamples, the peak integral of each sample was normalized. The number of CH3 groups is more or less the same in all samples. The CH3 aromatic is also the same in all samples. CH, compared to the control sample is a bit more in the 8 times sprayed sample, but a bit less in the 4 times sprayed sample. This suggests a change in the C= bands (Shankar, 2017). Control does not have any peaks at δ 4.7-5.5, therefore the number of CH groups is more in 4 and 8 times sprayed samples (and 8 times sprayed < 4 times sprayed sample).
The history of the invention, definition, method of producing mid-IR from MIRGA, toxicological analysis of MIRGA, safety of MIRGA sprayed primeval and usable, and potential applications of MIRGA have all been covered by [5,10,11] Detailed discussion in the supplementary file: Text T1.
While spraying MIRGA, most of the mid-IR energy scatters through the air and gets absorbed by receptors moringa leaf molecules. Virtually all organic compounds absorb mid-IR radiation which causes a change in the molecule’s vibrational state to move from the lower ground state to an excited higher energy state [12]. This leads to changes in chemical bonds [13,14] and these bond parameter changes lead to consequent changes in the target’s physical and chemical characters, configuration, and compound transformation depending on the dose of energy applied [8,15–17]. Nanostructured water layers can be triggered upon application of mid-IR radiation since water molecules absorb in this region [18,19].
The inorganic compounds that are employed to generate MIR offer a potential avenue for biomedical uses [20,21]. It's also a novel synthesis technique for producing functional material (2-6 µm mid-IR) [22,23]. It is common knowledge that combining various compounds with superior electronic properties creates new composite materials, which have attracted a lot of attention from the technology community recently [24,25].
In leafy vegetables, shelflife is the major drawback faced by consumers. Methods viz., pre-packing with different materials [29], chemical preservative application [30,31], freezing and relative humidity maintenance [32]. The results of these studies on shelflife are limited, moreover, gradual loss of sensory attributes is found to be inevitable. The methodology, labor, and expenses are more when compared to MIRGA technology. However, no studies have been performed so far to improve the taste and aroma of Moringa leaf. The MIRGA conveniently and concurrently enhanced the taste, aroma, and shelflife hence a present better alternative method.
In nature, the stereochemical configuration exerts a profound influence on the gustatory experience, as exemplified by the diverse flavors of mangoes, grapes, and rice. Boiling rice, transforming it into puffed rice, flattening it into flat rice, or converting it into rice flour result in distinct olfactory, gustatory, and textural qualities, as well as prolonged shelf-life, while maintaining a consistent molecular formula of C6H10O5. In the realm of the food industry, sensory attributes and shelf-life can be enhanced by manipulating the chemical bonds of food through various irradiation processes such as radappertization, radicidation, and radurization. Heating water leads to a sequential transition from solid ice to liquid water to gaseous steam, all of which are manifestations of alterations in hydrogen bonding, while the chemical composition of H2O remains unchanged. Similarly, by employing MIRGA, the chemical bonds in the moringa leaf molecules are altered to bring about the desirable taste and aroma.
We developed MIRGA with the intention of producing energy through a variety of processes. These processes include the ionization of atoms through spraying, resulting in the separation of electrons. This, in turn, creates multiple pathways for electron re-absorption, leading to the generation of energy. Additionally, during the spraying process, a water-based ionic solution becomes excited and charged, causing oscillation among the imbalanced ions in their excited state. As a result, photons are emitted. Furthermore, although a low electromagnetic field exists between the charged particles of the MIRGA's ionic solution, the induced oscillation between these particles during spraying produces energy. In the natural process of rainfall, more energy is needed to break the water bonds in order to create smaller water droplets. Consequently, these droplets possess more stored energy and gain kinetic energy as they travel downwards from a specific distance. When the raindrops make contact with the Earth's surface, they form a thin film of mid-IR (approximately 6 microns), resulting in a net heat gain. We replicated this energy-gaining process of rainfall in MIRGA. Specifically, when imbalanced ions in a liquid medium are atomized, the smaller droplets that are ejected should possess higher internal energy as well as acquired kinetic energy, in addition to the energy emitted by breaking the surface tension. Through trial and error, we adjusted the ejection pressure to achieve a desired fine mist and reduced the rate of evaporation by modifying the pH and density of the solution. Moreover, the accelerated ions within the sprayed ionic clouds collide with each other, generating energy. Therefore, we incorporated these phenomena into our atomizer and designed it in a manner that allows for the emission of energy in the mid-IR range of 2-6 µm, depending on the plunger pressure applied.
Depending on the number of MIRGA spraying (energy given), a receptor’s chemical bond configurations and subsequent physical and chemical characters can be altered to our desire. Such results obtained in coffee and tea, cocoa, and edible salts are presented by [5,10,11].
In the present study, instrumentation data analyses have shown that the mid-IR absorbed [19] by the organic compounds in moringa leaves has influenced the chemical bonds, thereby modifying the structure and altering the physico-chemical characteristics [26,27] through photostimulation and photobiomodulation [28].
In this study, Moringa leaves were irradiated with 2-6 µm mid-infrared. The irradiation enhanced the sensory attributes and shelf life of moringa leaves. This value addition has a chance to increase commercial demand. This technology can be extended to other leafy green vegetables to enhance their inherent characteristics.
By the journal’s policy and our ethical obligation as researchers, we submit that the authors Dr.Umakanthan and Dr.Madhu Mathi are the inventors and patentee of an Indian patent for MIRGA (under-patent no.: 401387) which is a major material employed in this study.
All data is available in the manuscript and supplementary materials.
The authors received no specific funding for this research.
Umakanthan: Conceptualization, Methodology, Project administration,
Madhu Mathi: Data curation, Investigation, Visualization, Writing - Original draft preparation.
Umadevi and Sivaramakrishnan: Resources, Supervision, Validation, Writing- Reviewing and Editing.
 |
| Figure 1: FTIR spectra of powdered moringa leaves |
 |
| Figure 2: PXRD spectra of powdered moringa leaves |
 |
| Figure 3: TEM - Electron diffraction patterns of powdered moringa leaves |
 |
| Figure 4: TEM - Bright field images of powdered moringa leaves |
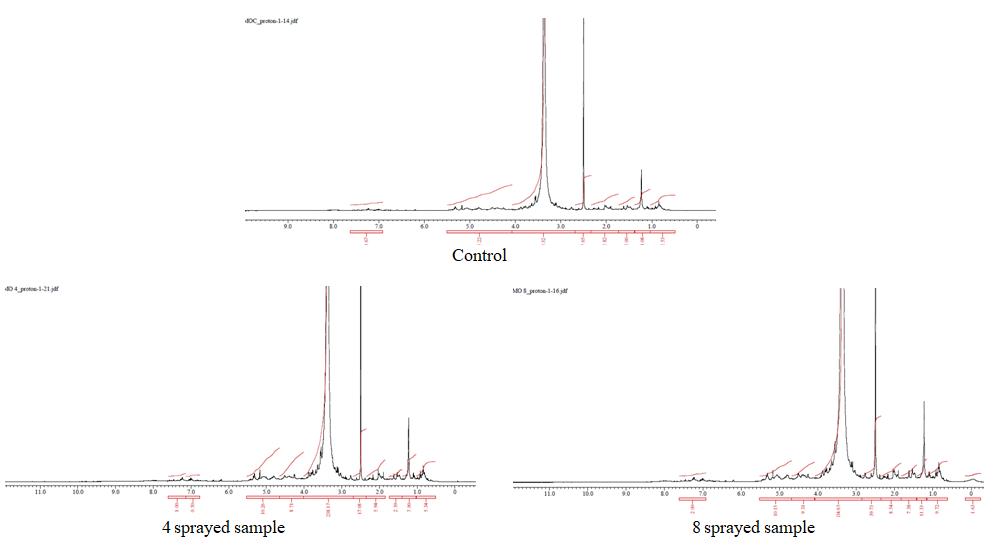 |
| Figure 5: 1H-NMR spectra of powdered moringa leaves |
Number of spraying |
Moringa leaves |
|
Taste |
Aroma |
|
Control |
5 |
5 |
1 |
3 |
4 |
2 |
5 |
6 |
3 |
7 |
7 |
4 |
9 |
8 |
5 |
7 |
6 |
6 |
5 |
3 |
7 |
2 |
2 |
8 |
1 |
1 |
Hedonic score scale: 1 - Dislike extremely, 2 - Dislike very much, 3 - Dislike moderately, 4 - Dislike slightly, 5 - Neither like nor dislike, 6 - Like slightly, 7 - Like moderately, 8 - Like very much, 9 - Like extremely
|
Control sample |
4 sprayed sample |
8 sprayed sample |
||||||
Chemical Shift (ppm) |
Peak Integral |
Normalized Peak Integral |
Chemical Shift (ppm) |
Peak Integral |
Normalized Peak Integral |
Chemical Shift (ppm) |
Peak Integral |
Normalized Peak Integral |
|
CH3 |
0.5-1.1 |
2.53 |
4 |
0.6-1.1 |
5.34 |
4 |
0.7-1.1 |
9.72 |
5 |
CH2 |
1.1-1.4 |
4.08 |
6 |
1.1-1.4 |
7.90 |
5 |
1.1-1.4 |
11.33 |
6 |
CH |
1.4-1.8 |
2.00 |
3 |
1.4-1.8 |
2.39 |
2 |
1.4-1.8 |
7.38 |
4 |
CH3 ar |
1.8-2.4 |
2.82 |
4 |
1.9- 2.4 |
5.95 |
4 |
1.8- 2.3 |
8.54 |
4 |
DMSO |
2.4-2.7 |
|
|
2.4-2.8 |
2.3-2.9 |
||||
H20 |
2.7-4.1 |
|
|
2.8-4.1 |
2.9-4.1 |
||||
CH |
4.1_5.5 |
5.22 |
8 |
4.1-4.7 |
8.71 |
6 |
4.1-4.7 |
9.31 |
5 |
CH |
--------- |
|
|
4.7- 5.5 |
10.26 |
7 |
4.7- 5.5 |
10.15 |
5 |
CH ar |
6.9-7.6 |
0.67 |
1 |
6.8-7.6 |
1.50 |
1 |
6.9-7.6 |
2.00 |
1 |






































